The [5+5] route to the phenanthrene skeleton
- PMID: 28286880
- PMCID: PMC5340207
- DOI: 10.3998/ark.5550190.p009.611
The [5+5] route to the phenanthrene skeleton
Abstract
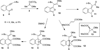
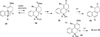
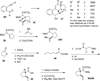
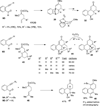
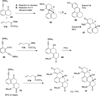
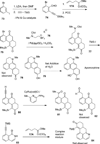
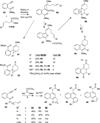
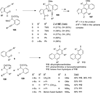
This account describes the historical development of the coupling of γ,δ-unsaturated Fischer carbene complexes and o-alkynylbenzaldehydes, which directly affords hydrophenanthrene ring systems in a process where each reactant contributes five carbons to the newly-formed bicyclo[4.4.0]decane ring system. The process has been termed net [5+5] cycloaddition. Use of the reaction to produce various medicinally important natural products and/or their parent ring systems is discussed.
Keywords: Diels-Alder reactions; Fischer carbene complexes; alkynes; annulation; cycloaddition; isobenzofurans; phenanthrenes.
Figures





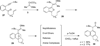










Similar articles
-
Fischer Carbene Complexes as Chemical Multitalents: The Incredible Range of Products from Carbenepentacarbonylmetal alpha,beta-Unsaturated Complexes.Angew Chem Int Ed Engl. 2000 Nov 17;39(22):3964-4002. doi: 10.1002/1521-3773(20001117)39:22<3964::aid-anie3964>3.0.co;2-c. Angew Chem Int Ed Engl. 2000. PMID: 11093193
-
Inversion of the direction of stereoinduction in the coupling of chiral gamma,delta-unsaturated Fischer carbene complexes with o-ethynylbenzaldehyde.Org Lett. 2002 Jun 27;4(13):2121-4. doi: 10.1021/ol025808y. Org Lett. 2002. PMID: 12074647
-
The first examples of a meta-benzannulation from the reaction of Fischer carbene complexes with alkynes.J Am Chem Soc. 2003 Jul 30;125(30):8980-1. doi: 10.1021/ja035428n. J Am Chem Soc. 2003. PMID: 15369331
-
New Methods and Strategies in the Synthesis of Terpenoid Natural Products.Acc Chem Res. 2021 Mar 16;54(6):1347-1359. doi: 10.1021/acs.accounts.0c00809. Epub 2021 Feb 17. Acc Chem Res. 2021. PMID: 33596652 Free PMC article. Review.
-
Triazines: Syntheses and Inverse Electron-demand Diels-Alder Reactions.Chem Rev. 2021 Dec 8;121(23):14555-14593. doi: 10.1021/acs.chemrev.1c00611. Epub 2021 Sep 29. Chem Rev. 2021. PMID: 34586777 Review.
Cited by
-
Synthesis of highly substituted benzene ring systems through three-component coupling of enyne imines, Fischer carbene complexes, and electron-deficient alkynes.Tetrahedron Lett. 2017 Apr 5;58(14):1403-1407. doi: 10.1016/j.tetlet.2017.02.070. Epub 2017 Feb 24. Tetrahedron Lett. 2017. PMID: 28966403 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
