Loss of Centromere Cohesion in Aneuploid Human Oocytes Correlates with Decreased Kinetochore Localization of the Sac Proteins Bub1 and Bubr1
- PMID: 28287092
- PMCID: PMC5347135
- DOI: 10.1038/srep44001
Loss of Centromere Cohesion in Aneuploid Human Oocytes Correlates with Decreased Kinetochore Localization of the Sac Proteins Bub1 and Bubr1
Abstract
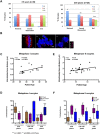
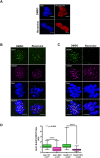
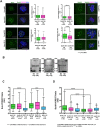
In human eggs, aneuploidy increases with age and can result in infertility and genetic diseases. Studies in mouse oocytes suggest that reduced centromere cohesion and spindle assembly checkpoint (SAC) activity could be at the origin of chromosome missegregation. Little is known about these two features in humans. Here, we show that in human eggs, inter-kinetochore distances of bivalent chromosomes strongly increase with age. This results in the formation of univalent chromosomes during metaphase I (MI) and of single chromatids in metaphase II (MII). We also investigated SAC activity by checking the localization of BUB1 and BUBR1. We found that they localize at the kinetochore with a similar temporal timing than in mitotic cells and in a MPS1-dependent manner, suggesting that the SAC signalling pathway is active in human oocytes. Moreover, our data also suggest that this checkpoint is inactivated when centromere cohesion is lost in MI and consequently cannot inhibit premature sister chromatid separation. Finally, we show that the kinetochore localization of BUB1 and BUBR1 decreases with the age of the oocyte donors. This could contribute to oocyte aneuploidy.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Haering C. H., Lowe J., Hochwagen A. & Nasmyth K. Molecular architecture of SMC proteins and the yeast cohesin complex. Molecular cell 9, 773–788 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

