Endogenous TNFα orchestrates the trafficking of neutrophils into and within lymphatic vessels during acute inflammation
- PMID: 28287124
- PMCID: PMC5347029
- DOI: 10.1038/srep44189
Endogenous TNFα orchestrates the trafficking of neutrophils into and within lymphatic vessels during acute inflammation
Abstract
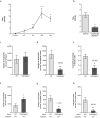
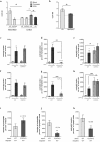
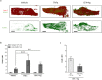
Neutrophils are recognised to play a pivotal role at the interface between innate and acquired immunities following their recruitment to inflamed tissues and lymphoid organs. While neutrophil trafficking through blood vessels has been extensively studied, the molecular mechanisms regulating their migration into the lymphatic system are still poorly understood. Here, we have analysed neutrophil-lymphatic vessel interactions in real time and in vivo using intravital confocal microscopy applied to inflamed cremaster muscles. We show that antigen sensitisation of the tissues induces a rapid but transient entry of tissue-infiltrated neutrophils into lymphatic vessels and subsequent crawling along the luminal side of the lymphatic endothelium. Interestingly, using mice deficient in both TNF receptors p55 and p75, chimeric animals and anti-TNFα antibody blockade we demonstrate that tissue-release of TNFα governs both neutrophil migration through the lymphatic endothelium and luminal crawling. Mechanistically, we show that TNFα primes directly the neutrophils to enter the lymphatic vessels in a strictly CCR7-dependent manner; and induces ICAM-1 up-regulation on lymphatic vessels, allowing neutrophils to crawl along the lumen of the lymphatic endothelium in an ICAM-1/MAC-1-dependent manner. Collectively, our findings demonstrate a new role for TNFα as a key regulator of neutrophil trafficking into and within lymphatic system in vivo.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

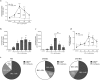


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

