Systematic Review and Network Meta-analysis of Idiopathic Pulmonary Fibrosis Treatments
- PMID: 28287346
- PMCID: PMC10410677
- DOI: 10.18553/jmcp.2017.23.3-b.s5
Systematic Review and Network Meta-analysis of Idiopathic Pulmonary Fibrosis Treatments
Abstract
Background: The antifibrotics pirfenidone and nintedanib are both approved for the treatment of idiopathic pulmonary fibrosis (IPF) by regulatory agencies and are recommended by health technology assessment bodies. Other treatments such as N-acetylcysteine are used in clinical practice but have not received regulatory approval. No head-to-head trials have been conducted to directly compare the efficacy of these therapies in IPF.
Objective: To compare the efficacy of treatments for IPF.
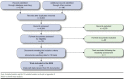
Methods: A systematic review was conducted up to April 2015. Phase II/III randomized controlled trials in adults with IPF were eligible. A Bayesian network meta-analysis (NMA) was used to compare pirfenidone, nintedanib, and N-acetylcysteine with respect to forced vital capacity (FVC) and mortality.
Results: Nine studies were included in the NMA. For change from baseline in FVC, the NMA indicated that pirfenidone and nintedanib were more effective than placebo after 1 year (pirfenidone vs. placebo: difference = 0.12 liter (L), 95% credible interval [CrI] = 0.03-0.21 L; nintedanib vs. placebo: difference = 0.11 L, 95% CrI = 0.00-0.22 L). There was no evidence that N-acetylcysteine had an effect on FVC compared with placebo (N-acetylcysteine vs. placebo: difference = 0.01 L, 95% CrI = -0.15-0.17 L). Patients treated with pirfenidone also had a lower risk of experiencing a decline in percent predicted FVC of ≥ 10% over 1 year (odds ratio [OR]: 0.58, 95% CrI = 0.40-0.88), whereas there was no conclusive evidence of a difference between nintedanib and placebo (OR: 0.65, 95% CrI = 0.42-1.02). The NMA indicated that pirfenidone reduced all-cause mortality relative to placebo over 1 year (hazard ratio [HR]: 0.52, 95% CrI = 0.28-0.92). There was no evidence of a difference in all-cause mortality between nintedanib and placebo (HR: 0.70, 95% CrI = 0.32-1.55), or N-acetylcysteine and placebo (HR: 2.00, 95% CrI=0.46-8.62).
Conclusions: Our primary analysis of the available evidence indicates that over 1 year, pirfenidone and nintedanib are effective at reducing lung-function decline, and pirfenidone may reduce the odds of experiencing a decline in percent predicted FVC of ≥10% compared with placebo in the first year of treatment. The results of our analysis also suggest that pirfenidone improves survival.
Disclosures: Fleetwood is an employee of Quantics Consulting. McCool and Glanville are employees of York Health Economics Consortium (YHEC). Quantics and YHEC received funding from F. Hoffmann-La Roche for conducting the systematic review and network meta-analysis reported in this paper. Edwards, Gsteiger, and Daigl are employees of F. Hoffmann-La Roche. Fisher was employed by InterMune UK, a wholly owned Roche subsidiary, until July 2015. He is currently employed by FIECON, which has received funding from F. Hoffmann-La Roche for consulting services. The systematic review and network meta-analysis reported in this paper were conducted by Fleetwood (Quantics Consulting) and McCool and Glanville (YHEC), funded by F. Hoffmann-La Roche. The original network analysis was funded by InterMune. Study concept and design were contributed by Edwards, Gsteiger, and Daigl, along with Fleetwood, McCool, and Glanville. Fleetwood, McCool, and Glanville collected the data, with assistance from Edwards, Gsteiger, and Daigl. Data interpretation was performed by Fleetwood and Fisher, with assistance from the other authors. The manuscript was written by Fleetwood, McCool, and Glanville, with assistance from Edwards, Daigl, and Fisher, and revised by all the authors.
Conflict of interest statement
Fleetwood is an employee of Quantics Consulting. McCool and Glanville are employees of York Health Economics Consortium (YHEC). Quantics and YHEC received funding from F. Hoffmann-La Roche for conducting the systematic review and network meta-analysis reported in this paper. Edwards, Gsteiger, and Daigl are employees of F. Hoffmann-La Roche. Fisher was employed by InterMune UK, a wholly owned Roche subsidiary, until July 2015. He is currently employed by FIECON, which has received funding from F. Hoffmann-La Roche for consulting services.
The systematic review and network meta-analysis reported in this paper were conducted by Fleetwood (Quantics Consulting) and McCool and Glanville (YHEC), funded by F. Hoffmann-La Roche. The original network analysis was funded by InterMune.
Study concept and design were contributed by Edwards, Gsteiger, and Daigl, along with Fleetwood, McCool, and Glanville. Fleetwood, McCool, and Glanville collected the data, with assistance from Edwards, Gsteiger, and Daigl. Data interpretation was performed by Fleetwood and Fisher, with assistance from the other authors. The manuscript was written by Fleetwood, McCool, and Glanville, with assistance from Edwards, Daigl, and Fisher, and revised by all the authors.
Figures
References
-
- European Medicines Agency. Public summary of opinion on orphan designation: pirfenidone for the treatment of idiopathic pulmonary fibrosis. March 12, 2015. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/.... Accessed February 9, 2017.
-
- Raghu G, Rochwerg B, Zhang Y, et al. .. An official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis: an update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015;192(2):e3-19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous