HDAC6-mediated acetylation of lipid droplet-binding protein CIDEC regulates fat-induced lipid storage
- PMID: 28287402
- PMCID: PMC5373879
- DOI: 10.1172/JCI85963
HDAC6-mediated acetylation of lipid droplet-binding protein CIDEC regulates fat-induced lipid storage
Abstract
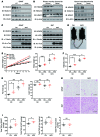
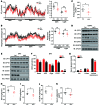
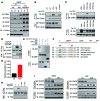
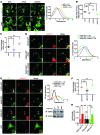
Obesity is characterized by aberrant fat accumulation. However, the intracellular signaling pathway that senses dietary fat and leads to fat storage remains elusive. Here, we have observed that the levels of histone deacetylase 6 (HDAC6) and the related family member HDAC10 are markedly reduced in adipose tissues of obese animals and humans. Mice with adipocyte-specific depletion of Hdac6 exhibited increased fat accumulation and reduced insulin sensitivity. In normal adipocytes, we found that reversal of P300/CBP-associated factor-induced (PCAF-induced) acetylation at K56 on cell death-inducing DFFA-like effector C (CIDEC, also known as FSP27) critically regulated lipid droplet fusion and lipid storage. Importantly, HDAC6 deacetylates CIDEC, leading to destabilization and reduced lipid droplet fusion. Accordingly, we observed elevated levels of CIDEC and its acetylated form in HDAC-deficient adipocytes as well as the adipose tissue of obese animals and humans. Fatty acids (FAs) prevented CIDEC deacetylation by promoting the dissociation of CIDEC from HDAC6, which resulted in increased association of CIDEC with PCAF on the endoplasmic reticulum. Control of CIDEC acetylation required the conversion of FAs to triacylglycerols. Thus, we have revealed a signaling axis that is involved in the coordination of nutrient availability, protein acetylation, and cellular lipid metabolic responses.
Conflict of interest statement
Figures



Similar articles
-
Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage.J Biol Chem. 2007 Nov 23;282(47):34213-8. doi: 10.1074/jbc.M707404200. Epub 2007 Sep 19. J Biol Chem. 2007. PMID: 17884815
-
Coordination Among Lipid Droplets, Peroxisomes, and Mitochondria Regulates Energy Expenditure Through the CIDE-ATGL-PPARα Pathway in Adipocytes.Diabetes. 2018 Oct;67(10):1935-1948. doi: 10.2337/db17-1452. Epub 2018 Jul 9. Diabetes. 2018. PMID: 29986925
-
Differential Roles of Cell Death-inducing DNA Fragmentation Factor-α-like Effector (CIDE) Proteins in Promoting Lipid Droplet Fusion and Growth in Subpopulations of Hepatocytes.J Biol Chem. 2016 Feb 26;291(9):4282-93. doi: 10.1074/jbc.M115.701094. Epub 2016 Jan 5. J Biol Chem. 2016. PMID: 26733203 Free PMC article.
-
Fat Cell and Fatty Acid Turnover in Obesity.Adv Exp Med Biol. 2017;960:135-160. doi: 10.1007/978-3-319-48382-5_6. Adv Exp Med Biol. 2017. PMID: 28585198 Review.
-
A physiological role for fat specific protein 27/cell death-inducing DFF45-like effector C in adipose and liver.Biol Pharm Bull. 2010;33(3):346-50. doi: 10.1248/bpb.33.346. Biol Pharm Bull. 2010. PMID: 20190390 Review.
Cited by
-
Protein acetylation: a novel modus of obesity regulation.J Mol Med (Berl). 2021 Sep;99(9):1221-1235. doi: 10.1007/s00109-021-02082-2. Epub 2021 Jun 1. J Mol Med (Berl). 2021. PMID: 34061242 Review.
-
First Fluorescent Acetylspermidine Deacetylation Assay for HDAC10 Identifies Selective Inhibitors with Cellular Target Engagement.Chembiochem. 2022 Jul 19;23(14):e202200180. doi: 10.1002/cbic.202200180. Epub 2022 Jun 10. Chembiochem. 2022. PMID: 35608330 Free PMC article.
-
Histone deacetylase 6 inhibition restores leptin sensitivity and reduces obesity.Nat Metab. 2022 Jan;4(1):44-59. doi: 10.1038/s42255-021-00515-3. Epub 2022 Jan 17. Nat Metab. 2022. PMID: 35039672 Free PMC article.
-
Shift work promotes adipogenesis via cortisol-dependent downregulation of EGR3-HDAC6 pathway.Cell Death Discov. 2024 Mar 11;10(1):129. doi: 10.1038/s41420-024-01904-9. Cell Death Discov. 2024. PMID: 38467615 Free PMC article.
-
Rab18 maintains homeostasis of subcutaneous adipose tissue to prevent obesity-induced metabolic disorders.Sci China Life Sci. 2024 Jun;67(6):1170-1182. doi: 10.1007/s11427-023-2367-9. Epub 2024 Mar 22. Sci China Life Sci. 2024. PMID: 38523235
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

