High Hydrostatic Pressure (HHP)-Induced Structural Modification of Patatin and Its Antioxidant Activities
- PMID: 28287443
- PMCID: PMC6155260
- DOI: 10.3390/molecules22030438
High Hydrostatic Pressure (HHP)-Induced Structural Modification of Patatin and Its Antioxidant Activities
Abstract
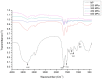
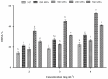
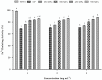
Patatin represents a group of homologous primary storage proteins (with molecular weights ranging from 40 kDa to 45 kDa) found in Solanum tuberosum L. This group comprises 40% of the total soluble proteins in potato tubers. Here, patatin (40 kDa) was extracted from potato fruit juice using ammonium sulfate precipitation (ASP) and exposed to high hydrostatic pressure (HHP) treatment (250, 350, 450, and 550 MPa). We investigated the effect of HHP treatment on the structure, composition, heat profile, and antioxidant potential, observing prominent changes in HHP-induced patatin secondary structure as compared with native patatin (NP). Additionally, significant (p < 0.05) increases in β-sheet content along with decreases in α-helix content were observed following HHP treatment. Thermal changes observed by differential scanning calorimetry (DSC) also showed a similar trend following HHP treatment; however, the enthalpy of patatin was also negatively affected by pressurization, and free sulfhydryl content and surface hydrophobicity significantly increased with pressurization up to 450 MPa, although both interactions progressively decreased at 550 MPa. The observed physicochemical changes suggested conformational modifications in patatin induced by HHP treatment. Moreover, our results indicated marked enhancement of antioxidant potential, as well as iron chelation activities, in HHP-treated patatin as compared with NP. These results suggested that HHP treatment offers an effective and green process for inducing structural modifications and improving patatin functionality.
Keywords: antioxidant activities; high hydrostatic pressure; iron chelation potential; potato patatin; surface hydrophobicity; thermal properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Patatin, the tuber storage protein of potato (Solanum tuberosum L.), exhibits antioxidant activity in vitro.J Agric Food Chem. 2003 Jul 16;51(15):4389-93. doi: 10.1021/jf030016j. J Agric Food Chem. 2003. PMID: 12848515
-
Effects of high hydrostatic pressure on physicochemical and functional properties of walnut (Juglans regia L.) protein isolate.J Sci Food Agric. 2013 Mar 30;93(5):1105-11. doi: 10.1002/jsfa.5857. Epub 2012 Aug 30. J Sci Food Agric. 2013. PMID: 22936330
-
Extraction of chitosan from squid pen waste by high hydrostatic pressure: Effects on physicochemical properties and antioxidant activities of chitosan.Int J Biol Macromol. 2020 Oct 1;160:677-687. doi: 10.1016/j.ijbiomac.2020.05.252. Epub 2020 May 30. Int J Biol Macromol. 2020. PMID: 32479945
-
Antifungal and antimicrobial proteins and peptides of potato (Solanum tuberosum L.) tubers and their applications.Appl Microbiol Biotechnol. 2019 Jul;103(14):5533-5547. doi: 10.1007/s00253-019-09887-9. Epub 2019 May 29. Appl Microbiol Biotechnol. 2019. PMID: 31144014 Review.
-
Factors affecting the modification of bovine milk proteins in high hydrostatic pressure processing: An updated review.Compr Rev Food Sci Food Saf. 2022 Sep;21(5):4274-4293. doi: 10.1111/1541-4337.13012. Epub 2022 Jul 29. Compr Rev Food Sci Food Saf. 2022. PMID: 35904187 Review.
Cited by
-
High hydrostatic pressure (HHP) effects on antigenicity and structural properties of soybean β-conglycinin.J Food Sci Technol. 2018 Feb;55(2):630-637. doi: 10.1007/s13197-017-2972-2. Epub 2017 Nov 25. J Food Sci Technol. 2018. PMID: 29391627 Free PMC article.
-
Impact of the Structural Modifications of Potato Protein in the Digestibility Process under Semi-Dynamic Simulated Human Gastrointestinal In Vitro System.Nutrients. 2022 Jun 16;14(12):2505. doi: 10.3390/nu14122505. Nutrients. 2022. PMID: 35745236 Free PMC article.
-
High Hydrostatic Pressure: Influences on Allergenicity, Bioactivities, and Structural and Functional Properties of Proteins from Diverse Food Sources.Foods. 2024 Mar 18;13(6):922. doi: 10.3390/foods13060922. Foods. 2024. PMID: 38540912 Free PMC article. Review.
-
Comparison of Thermal and High-Pressure Gelation of Potato Protein Isolates.Foods. 2020 Aug 2;9(8):1041. doi: 10.3390/foods9081041. Foods. 2020. PMID: 32748833 Free PMC article.
-
Integrated Efforts for the Valorization of Sweet Potato By-Products within a Circular Economy Concept: Biocomposites for Packaging Applications Close the Loop.Polymers (Basel). 2021 Mar 27;13(7):1048. doi: 10.3390/polym13071048. Polymers (Basel). 2021. PMID: 33801582 Free PMC article.
References
-
- FAOSTAT . Food and Nutrition. FAO; Rome, Italy: 2014.
-
- Du-qin Z., Tai-hua M.U., Hong-nan S.U.N. Domestic and Abroad Research Progress of Potato Tuber-Specific Storage Protein Patatin. Sci. Agric. Sin. 2016;49:1746–1756.
-
- Pots A.M., Gruppen H., Van Diepenbeek R., Van Der Lee J.J., Van Boekel M.A.J.S., Wijngaards G., Voragen A.G.J. The effect of storage of whole potatoes of three cultivars on the patatin and protease inhibitor content; a study using capillary electrophoresis and MALDI-TOF mass spectrometry. J. Sci. Food Agric. 1999;79:1557–1564. doi: 10.1002/(SICI)1097-0010(199909)79:12<1557::AID-JSFA375>3.0.CO;2-K. - DOI
-
- Bárta J., Bártová V. Patatin, the major protein of potato (Solanum tuberosum L.) tubers, and its occurrence as genotype effect: Processing versus table potatoes. Czech J. Food Sci. 2008;26:347–359.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

