Strawberry-Tree Honey Induces Growth Inhibition of Human Colon Cancer Cells and Increases ROS Generation: A Comparison with Manuka Honey
- PMID: 28287469
- PMCID: PMC5372629
- DOI: 10.3390/ijms18030613
Strawberry-Tree Honey Induces Growth Inhibition of Human Colon Cancer Cells and Increases ROS Generation: A Comparison with Manuka Honey
Abstract
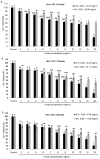
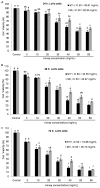
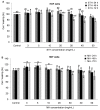
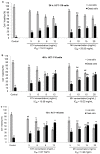
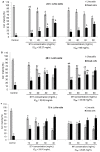
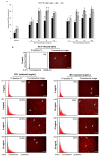
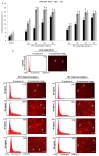
Honey is a natural product known to modulate several biological activities including cancer. The aim of the present study was to examine the phytochemical content and the antioxidant activity of Strawberry tree (Arbutus unedo) honey (STH) and its cytotoxic properties against human colon adenocarcinoma (HCT-116) and metastatic (LoVo) cell lines in comparison with Manuka (Leptospermum scoparium) honey (MH). Several unifloral STH and MH were analyzed for their phenolic, flavonoid, amino acid and protein contents, as well as their radical scavenging activities. STH from the Berchidda area showed the highest amount of phenolic, flavonoid, amino acid and protein content, and antioxidant capacity compared to MH. Both STH and MH induced cytotoxicity and cell death in a dose- and time-dependent manner in HCT-116 and LoVo cells, with less toxicity on non-cancer cells. Compared to MH, STH showed more effect at lower concentrations on HCT-116 and LoVo cells. In addition, both honeys increased intracellular reactive oxygen species (ROS) generation. In HCT-116 cells, STH and MH induced similar ROS production but in LoVo cells STH induced a higher percentage of ROS compared to MH. Our results indicate that STH and MH can induce cell growth inhibition and ROS generation in colon adenocarcinoma and metastatic cells, which could be due to the presence of phytochemicals with antioxidant properties. These preliminary results are interesting and suggest a potential chemopreventive action which could be useful for further studies in order to develop chemopreventive agents for colon cancer.
Keywords: Manuka honey; antioxidant activity; colon cancer; cytotoxicity; polyphenols; reactive oxygen species; strawberry tree honey.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Afrin S., Giampieri F., Gasparrini M., Forbes-Hernandez T.Y., Varela-López A., Quiles J.L., Mezzetti B., Battino M. Chemopreventive and therapeutic effects of edible berries: A focus on colon cancer prevention and treatment. Molecules. 2016;21:169. doi: 10.3390/molecules21020169. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

