Effects of adolescent exposure to methylmercury and d-amphetamine on reversal learning and an extradimensional shift in male mice
- PMID: 28287789
- PMCID: PMC5367946
- DOI: 10.1037/pha0000107
Effects of adolescent exposure to methylmercury and d-amphetamine on reversal learning and an extradimensional shift in male mice
Abstract
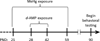
Adolescence is associated with the continued maturation of dopamine neurotransmission and is implicated in the etiology of many psychiatric illnesses. Adolescent exposure to neurotoxicants that distort dopamine neurotransmission, such as methylmercury (MeHg), may modify the effects of chronic d-amphetamine (d-AMP) administration on reversal learning and attentional-set shifting. Male C57Bl/6n mice were randomly assigned to two MeHg-exposure groups (0 ppm and 3 ppm) and two d-AMP-exposure groups (saline and 1 mg/kg/day), producing four treatment groups (n = 10-12/group): control, MeHg, d-AMP, and MeHg + d-AMP. MeHg exposure (via drinking water) spanned postnatal days 21-59 (the murine adolescent period), and once daily intraperitoneal injections of d-AMP or saline spanned postnatal days 28-42. As adults, mice were trained on a spatial-discrimination-reversal (SDR) task in which the spatial location of a lever press predicted reinforcement. Following 2 SDRs, a visual-discrimination task (extradimensional shift) was instated in which the presence of a stimulus light above a lever predicted reinforcement. Responding was modeled using a logistic function, which estimated the rate (slope) of a behavioral transition and trials required to complete half a transition (half-max). MeHg, d-AMP, and MeHg + d-AMP exposure increased estimates of half-max on the second reversal. MeHg exposure increased half-max and decreased the slope term following the extradimensional shift, but these effects did not occur following MeHg + d-AMP exposure. MeHg + d-AMP exposure produced more perseverative errors and omissions following a reversal. Adolescent exposure to MeHg can modify the behavioral effects of chronic d-AMP administration. (PsycINFO Database Record
(c) 2017 APA, all rights reserved).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


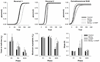
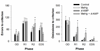
References
-
- Adriani W, Macrì S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27(2):212–224. - PubMed
-
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews. 2003;27(1–2):3–18. http://doi.org/10.1016/S0149-7634(03)00005-8. - DOI - PubMed
-
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8(6):1495–1498. http://doi.org/10.1097/00001756-199704140-00034. - DOI - PubMed
-
- Andersen SL, Thompson A, Rutstein M, Hostetter J, Teicher M. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37(2):167–169. http://doi.org/10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. - DOI - PubMed
-
- Arnsten AFT, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: Disruptions in neurodevelopmental psychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(4):356–367. http://doi.org/10.1016/j.jaac.2012.01.008. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

