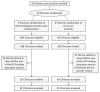
An Electronic Health Record-based Intervention to Promote Hepatitis C Virus Testing Among Adults Born Between 1945 and 1965: A Cluster-randomized Trial
- PMID: 28288075
- PMCID: PMC5759753
- DOI: 10.1097/MLR.0000000000000715
An Electronic Health Record-based Intervention to Promote Hepatitis C Virus Testing Among Adults Born Between 1945 and 1965: A Cluster-randomized Trial
Abstract
Background: The Centers for Disease Control and Prevention (CDC) recommends one-time hepatitis C virus (HCV) antibody testing for "Birth Cohort" adults born during 1945-1965.
Objective: To examine the impact of an electronic health record (EHR)-embedded best practice alert (BPA) for HCV testing among Birth Cohort adults.
Design: Cluster-randomized trial was conducted from April 29, 2013 to March 29, 2014.
Subjects and setting: Ten community and hospital-based primary care practices. Participants were attending physicians and medical residents during 25,620 study-eligible visits.
Intervention: Physicians in all practices received a brief introduction to the CDC testing recommendations. At visits for eligible patients at intervention sites, physicians received a BPA through the EHR to order HCV testing or medical assistants were prompted to post a testing order for the physician. Physicians in control sites did not receive the BPA.
Main outcomes: HCV testing; the incidence of HCV antibody positive tests was a secondary outcome.
Results: Testing rates were greater among Birth Cohort patients in intervention sites (20.2% vs. 1.8%, P<0.0001) and the odds of testing were greater in intervention sites after controlling for imbalances of patient and visit characteristics between comparison groups [odds ratio (OR), 9.0; 95% confidence interval, 7.6-10.7). The adjusted OR of identifying HCV antibody positive patients was also greater in intervention sites (OR, 2.1; 95% confidence interval, 1.3-11.2).
Conclusions: An EHR-embedded BPA markedly increased HCV testing among Birth Cohort patients, but the majority of eligible patients did not receive testing indicating a need for more effective methods to promote uptake.
Trial registration: ClinicalTrials.gov NCT02123212.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
An Electronic Health Record-based Intervention to Promote Hepatitis C Virus Testing Among Adults Born Between 1945 and 1965: A Cluster-randomized Trial.Med Care. 2018 Mar;56(3):274. doi: 10.1097/MLR.0000000000000872. Med Care. 2018. PMID: 29309393 No abstract available.
-
Author Response for An Electronic Health Record-based Intervention to Promote Hepatitis C Virus Testing Among Adults Born Between 1945 and 1965: A Cluster-randomized Trial.Med Care. 2018 Mar;56(3):274. doi: 10.1097/MLR.0000000000000873. Med Care. 2018. PMID: 29329149 No abstract available.
Similar articles
-
Improving Hepatitis C Virus Screening Rates in Primary Care: A Targeted Intervention Using the Electronic Health Record.J Healthc Qual. 2015 Sep-Oct;37(5):319-23. doi: 10.1097/JHQ.0000000000000010. J Healthc Qual. 2015. PMID: 26186704
-
Primary care-based interventions are associated with increases in hepatitis C virus testing for patients at risk.Dig Liver Dis. 2012 Jun;44(6):497-503. doi: 10.1016/j.dld.2011.12.014. Epub 2012 Feb 18. Dig Liver Dis. 2012. PMID: 22342471
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Impact of an electronic health record alert in primary care on increasing hepatitis c screening and curative treatment for baby boomers.Hepatology. 2017 Dec;66(6):1805-1813. doi: 10.1002/hep.29362. Epub 2017 Sep 14. Hepatology. 2017. PMID: 28714196 Free PMC article.
-
Comparing Ways to Increase Hepatitis B and C Screening among Asian Americans [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Feb. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Feb. PMID: 39808005 Free Books & Documents. Review.
Cited by
-
Patient and provider-level barriers to hepatitis C screening and linkage to care: A mixed-methods evaluation.J Viral Hepat. 2020 Jul;27(7):680-689. doi: 10.1111/jvh.13278. Epub 2020 Mar 2. J Viral Hepat. 2020. PMID: 32048397 Free PMC article.
-
Developing an Ophthalmology Clinical Decision Support System to Identify Patients for Low Vision Rehabilitation.Transl Vis Sci Technol. 2021 Mar 1;10(3):24. doi: 10.1167/tvst.10.3.24. Transl Vis Sci Technol. 2021. PMID: 34003955 Free PMC article.
-
Differences in inpatient and outpatient hepatitis C virus prevalence and linkage to care rates in a safety net hospital hepatitis C screening program.J Gastroenterol Hepatol. 2021 Aug;36(8):2285-2291. doi: 10.1111/jgh.15492. Epub 2021 Mar 31. J Gastroenterol Hepatol. 2021. PMID: 33724551 Free PMC article.
-
Hepatitis C Virus Screening: Factors Associated With Test Completion in a Large Academic Health Care System.Public Health Rep. 2022 Nov-Dec;137(6):1136-1145. doi: 10.1177/00333549211054085. Epub 2021 Oct 25. Public Health Rep. 2022. PMID: 34694928 Free PMC article.
-
Assessing the Effectiveness of Strategies in US Birth Cohort Screening for Hepatitis C Infection.J Clin Transl Hepatol. 2020 Mar 28;8(1):25-41. doi: 10.14218/JCTH.2019.00059. Epub 2020 Mar 24. J Clin Transl Hepatol. 2020. PMID: 32274343 Free PMC article. Review.
References
-
- United States Preventive Services Task Force. Hepatitis C screening. [Accessed May 3, 2016];Recommendation summary. 2013 Available at: www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/....
-
- New York State Department of Health. [Accessed May 3, 2016];NYS hepatitis C testing law: frequently asked questions. 2014 Available at: www.health.ny.gov/diseases/communicable/hepatitis/hepatitis_c/rapid_anti....
-
- McGarry LJ, Pawar VS, Panchmatia HR, et al. Economic model of a birth cohort screening program for hepatitis C virus. Hepatology. 2012;55:1344–1355. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous