Germline mutations in ABL1 cause an autosomal dominant syndrome characterized by congenital heart defects and skeletal malformations
- PMID: 28288113
- PMCID: PMC5373987
- DOI: 10.1038/ng.3815
Germline mutations in ABL1 cause an autosomal dominant syndrome characterized by congenital heart defects and skeletal malformations
Abstract
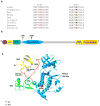
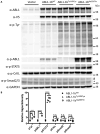
ABL1 is a proto-oncogene well known as part of the fusion gene BCR-ABL1 in the Philadelphia chromosome of leukemia cancer cells. Inherited germline ABL1 changes have not been associated with genetic disorders. Here we report ABL1 germline variants cosegregating with an autosomal dominant disorder characterized by congenital heart disease, skeletal abnormalities, and failure to thrive. The variant c.734A>G (p.Tyr245Cys) was found to occur de novo or cosegregate with disease in five individuals (families 1-3). Additionally, a de novo c.1066G>A (p.Ala356Thr) variant was identified in a sixth individual (family 4). We overexpressed the mutant constructs in HEK 293T cells and observed increased tyrosine phosphorylation, suggesting increased ABL1 kinase activities associated with both the p.Tyr245Cys and p.Ala356Thr substitutions. Our clinical and experimental findings, together with previously reported teratogenic effects of selective BCR-ABL inhibitors in humans and developmental defects in Abl1 knockout mice, suggest that ABL1 has an important role during organismal development.
Figures


References
-
- de Klein A, et al. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300:765–7. - PubMed
-
- Apperley J. Issues of imatinib and pregnancy outcome. J Natl Compr Canc Netw. 2009;7:1050–8. - PubMed
-
- Ault P, et al. Pregnancy among patients with chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2006;24:1204–8. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

