Genetically encoded biosensors for visualizing live-cell biochemical activity at super-resolution
- PMID: 28288122
- PMCID: PMC5388356
- DOI: 10.1038/nmeth.4221
Genetically encoded biosensors for visualizing live-cell biochemical activity at super-resolution
Abstract
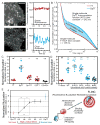
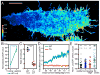
Compartmentalized biochemical activities are essential to all cellular processes, but there is no generalizable method to visualize dynamic protein activities in living cells at a resolution commensurate with cellular compartmentalization. Here, we introduce a new class of fluorescent biosensors that detect biochemical activities in living cells at a resolution up to threefold better than the diffraction limit. These 'FLINC' biosensors use binding-induced changes in protein fluorescence dynamics to translate kinase activities or protein-protein interactions into changes in fluorescence fluctuations, which are quantifiable through stochastic optical fluctuation imaging. A protein kinase A (PKA) biosensor allowed us to resolve minute PKA activity microdomains on the plasma membranes of living cells and to uncover the role of clustered anchoring proteins in organizing these activity microdomains. Together, these findings suggest that biochemical activities of the cell are spatially organized into an activity architecture whose structural and functional characteristics can be revealed by these new biosensors.
Figures



References
-
- Avraham R, Yarden Y. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nature Reviews Molecular Cell Biology. 2011;12:104–117. - PubMed
-
- Wong W, Scott JD. AKAP signalling complexes: Focal points in space and time. Nature Reviews Molecular Cell Biology. 2004;5:959–970. - PubMed
-
- Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: Molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. - PubMed
METHODS-ONLY REFERENCES
-
- Manna P, Jimenez R. Time and Frequency-Domain Measurement of Ground-State Recovery Times in Red Fluorescent Proteins. Journal of Physical Chemistry B. 2015;119:4944–4954. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

