Serine hydroxymethyl transferase 1 stimulates pro-oncogenic cytokine expression through sialic acid to promote ovarian cancer tumor growth and progression
- PMID: 28288142
- PMCID: PMC5509519
- DOI: 10.1038/onc.2017.37
Serine hydroxymethyl transferase 1 stimulates pro-oncogenic cytokine expression through sialic acid to promote ovarian cancer tumor growth and progression
Abstract
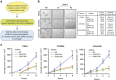
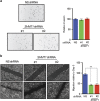
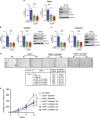
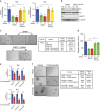
High-grade serous (HGS) ovarian cancer accounts for 90% of all ovarian cancer-related deaths. However, factors that drive HGS ovarian cancer tumor growth have not been fully elucidated. In particular, comprehensive analysis of the metabolic requirements of ovarian cancer tumor growth has not been performed. By analyzing The Cancer Genome Atlas mRNA expression data for HGS ovarian cancer patient samples, we observed that six enzymes of the folic acid metabolic pathway were overexpressed in HGS ovarian cancer samples compared with normal ovary samples. Systematic knockdown of all six genes using short hairpin RNAs (shRNAs) and follow-up functional studies demonstrated that serine hydroxymethyl transferase 1 (SHMT1) was necessary for ovarian cancer tumor growth and cell migration in culture and tumor formation in mice. SHMT1 promoter analysis identified transcription factor Wilms tumor 1 (WT1) binding sites, and WT1 knockdown resulted in reduced SHMT1 transcription in ovarian cancer cells. Unbiased large-scale metabolomic analysis and transcriptome-wide mRNA expression profiling identified reduced levels of several metabolites of the amino sugar and nucleotide sugar metabolic pathways, including sialic acid N-acetylneuraminic acid (Neu5Ac), and downregulation of pro-oncogenic cytokines interleukin-6 and 8 (IL-6 and IL-8) as unexpected outcomes of SHMT1 loss. Overexpression of either IL-6 or IL-8 partially rescued SHMT1 loss-induced tumor growth inhibition and migration. Supplementation of culture medium with Neu5Ac stimulated expression of IL-6 and IL-8 and rescued the tumor growth and migratory phenotypes of ovarian cancer cells expressing SHMT1 shRNAs. In agreement with the ovarian tumor-promoting role of Neu5Ac, treatment with Neu5Ac-targeting glycomimetic P-3Fax-Neu5Ac blocked ovarian cancer growth and migration. Collectively, these results demonstrate that SHMT1 controls the expression of pro-oncogenic inflammatory cytokines by regulating sialic acid Neu5Ac to promote ovarian cancer tumor growth and migration. Thus, targeting of SHMT1 and Neu5Ac represents a precision therapy opportunity for effective HGS ovarian cancer treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Systematic analysis reveals a lncRNA-mRNA co-expression network associated with platinum resistance in high-grade serous ovarian cancer.Invest New Drugs. 2018 Apr;36(2):187-194. doi: 10.1007/s10637-017-0523-3. Epub 2017 Oct 30. Invest New Drugs. 2018. PMID: 29082457
-
Pericytes Promote Malignant Ovarian Cancer Progression in Mice and Predict Poor Prognosis in Serous Ovarian Cancer Patients.Clin Cancer Res. 2016 Apr 1;22(7):1813-24. doi: 10.1158/1078-0432.CCR-15-1931. Epub 2015 Nov 20. Clin Cancer Res. 2016. PMID: 26589433
-
Targeting miR-21-3p inhibits proliferation and invasion of ovarian cancer cells.Oncotarget. 2016 Jun 14;7(24):36321-36337. doi: 10.18632/oncotarget.9216. Oncotarget. 2016. PMID: 27166999 Free PMC article.
-
Role of Increased n-acetylaspartate Levels in Cancer.J Natl Cancer Inst. 2016 Jan 26;108(6):djv426. doi: 10.1093/jnci/djv426. J Natl Cancer Inst. 2016. PMID: 26819345 Free PMC article.
-
[Analysis of mouse strain-dependent prepulse inhibition points to a role for Shmt1 (SHMT1) in mice and in schizophrenia].Nihon Shinkei Seishin Yakurigaku Zasshi. 2010 Nov;30(5-6):197-200. Nihon Shinkei Seishin Yakurigaku Zasshi. 2010. PMID: 21226315 Review. Japanese.
Cited by
-
Sialic acids in gynecological cancer development and progression: Impact on diagnosis and treatment.Int J Cancer. 2022 Feb 15;150(4):678-687. doi: 10.1002/ijc.33866. Epub 2021 Nov 17. Int J Cancer. 2022. PMID: 34741527 Free PMC article. Review.
-
The moonlighting RNA-binding activity of cytosolic serine hydroxymethyltransferase contributes to control compartmentalization of serine metabolism.Nucleic Acids Res. 2019 May 7;47(8):4240-4254. doi: 10.1093/nar/gkz129. Nucleic Acids Res. 2019. PMID: 30809670 Free PMC article.
-
HOXD8 suppresses renal cell carcinoma growth by upregulating SHMT1 expression.Cancer Sci. 2023 Dec;114(12):4583-4595. doi: 10.1111/cas.15982. Epub 2023 Sep 26. Cancer Sci. 2023. PMID: 37752684 Free PMC article.
-
Metabolic reprogramming of three major nutrients in platinum-resistant ovarian cancer.Front Oncol. 2023 Aug 22;13:1231460. doi: 10.3389/fonc.2023.1231460. eCollection 2023. Front Oncol. 2023. PMID: 37681030 Free PMC article. Review.
-
Serine and glycine metabolism-related gene expression signature stratifies immune profiles of brain gliomas, and predicts prognosis and responses to immunotherapy.Front Pharmacol. 2022 Nov 17;13:1072253. doi: 10.3389/fphar.2022.1072253. eCollection 2022. Front Pharmacol. 2022. PMID: 36467068 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. - PubMed
-
- Cannistra SA. Cancer of the ovary. N Engl J Med 2004; 351: 2519–2529. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. - PubMed
-
- Miranda F, Mannion D, Liu S, Zheng Y, Mangala LS, Redondo C et al. Salt-inducible kinase 2 couples ovarian cancer cell metabolism with survival at the adipocyte-rich metastatic niche. Cancer Cell 2016; 30: 273–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

