Antioxidant Capacity and Superoxide Dismutase Activity in Adrenoleukodystrophy
- PMID: 28288261
- PMCID: PMC5822206
- DOI: 10.1001/jamaneurol.2016.5715
Antioxidant Capacity and Superoxide Dismutase Activity in Adrenoleukodystrophy
Abstract
Importance: X-linked adrenoleukodystrophy (ALD) may switch phenotype to the fatal cerebral form (ie, cerebral ALD [cALD]), the cause of which is unknown. Determining differences in antioxidant capacity and superoxide dismutase (SOD) levels between phenotypes may allow for the generation of a clinical biomarker for predicting the onset of cALD, as well as initiating a more timely lifesaving therapy.
Objective: To identify variations in the levels of antioxidant capacity and SOD activity between ALD phenotypes in patients with cALD or adrenomyeloneuropathy (AMN), heterozygote female carriers, and healthy controls and, in addition, correlate antioxidant levels with clinical outcome scores to determine a possible predictive value.
Design, setting, and participants: Samples of monocytes and blood plasma were prospectively collected from healthy controls, heterozygote female carriers, and patients with AMN or cALD. We are counting each patient as 1 sample in our study. Because adrenoleukodystrophy is an X-linked disease, the affected group populations of cALD and AMN are all male. The heterozygote carriers are all female. The samples were assayed for total antioxidant capacity and SOD activity. The data were collected in an academic hospital setting. Eligibility criteria included patients who received a diagnosis of ALD and heterozygote female carriers, both of which groups were compared with age-matched controls. The prospective samples (n = 30) were collected between January 2015 to January 2016, and existing samples were collected from tissue storage banks at the Kennedy Krieger Institute (n = 30). The analyses were performed during the first 3 months of 2016.
Main outcome and measures: Commercially available total antioxidant capacity and SOD assays were performed on samples of monocytes and blood plasma and correlated with magnetic resonance imaging severity score.
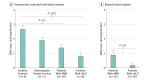
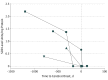
Results: A reduction in antioxidant capacity was shown between the healthy controls (0.225 mmol trolox equivalent) and heterozygote carriers (0.181 mmol trolox equivalent), and significant reductions were seen between healthy controls and patients with AMN (0.102 mmol trolox equivalent; P < .01), as well as healthy controls and patients with cALD (0.042 mmol trolox equivalent; P < .01). Superoxide dismutase activity in human blood plasma mirrored these reductions between prospectively collected samples from healthy controls (2.66 units/mg protein) and samples from heterozygote female carriers (1.91 units/mg protein), patients with AMN (1.39 units/mg protein; P = .01), and patients with cALD (0.8 units/mg protein; P < .01). Further analysis of SOD activity in biobank samples showed significant reductions between patients with AMN (0.89 units/mg protein) and patients with cALD (0.18 units/mg protein) (P = .03). Plasma SOD levels from patients with cALD demonstrated an inverse correlation to brain magnetic resonance imaging severity score (R2 = 0.75, P < .002). Longitudinal plasma SOD samples from the same patients (n = 4) showed decreased activity prior to and at the time of cerebral diagnosis over a period of 13 to 42 months (mean period, 24 months).
Conclusions and relevance: Plasma SOD may serve as a potential biomarker for cerebral disease in ALD following future prospective studies.
Conflict of interest statement
Figures


References
-
- Moser HW, Mahmood A, Raymond GV. X-linked adrenoleukodystrophy. Nat Clin Pract Neurol. 2007;3(3):140-151. - PubMed
-
- van Roermund CWT, Visser WF, Ijlst L, et al. . The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 2008;22(12):4201-4208. - PubMed
-
- O’Neill GN, Aoki M, Brown RH Jr. ABCD1 translation-initiator mutation demonstrates genotype-phenotype correlation for AMN. Neurology. 2001;57(11):1956-1962. - PubMed
-
- Engelen M, Kemp S, Poll-The BT. X-linked adrenoleukodystrophy: pathogenesis and treatment. Curr Neurol Neurosci Rep. 2014;14(10):486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

