Effect of tolvaptan on renal water and sodium excretion and blood pressure during nitric oxide inhibition: a dose-response study in healthy subjects
- PMID: 28288570
- PMCID: PMC5347830
- DOI: 10.1186/s12882-017-0501-1
Effect of tolvaptan on renal water and sodium excretion and blood pressure during nitric oxide inhibition: a dose-response study in healthy subjects
Abstract
Background: Tolvaptan is a selective vasopressin receptor antagonist. Nitric Oxide (NO) promotes renal water and sodium excretion, but the effect is unknown in the nephron's principal cells. In a dose-response study, we measured the effect of tolvaptan on renal handling of water and sodium and systemic hemodynamics, during baseline and NO-inhibition with L-NMMA (L-NG-monomethyl-arginine).
Methods: In a randomized, placebo-controlled, double blind, cross over study, 15 healthy subjects received tolvaptan 15, 30 and 45 mg or placebo. L-NMMA was given as a bolus followed by continuous infusion during 60 min. We measured urine output (UO), free water clearance (CH2O), fractional excretion of sodium (FENa), urinary aquaporin-2 channels (u-AQP2) and epithelial sodium channels (u-ENaCγ), plasma vasopressin (p-AVP) and central blood pressure (cBP).
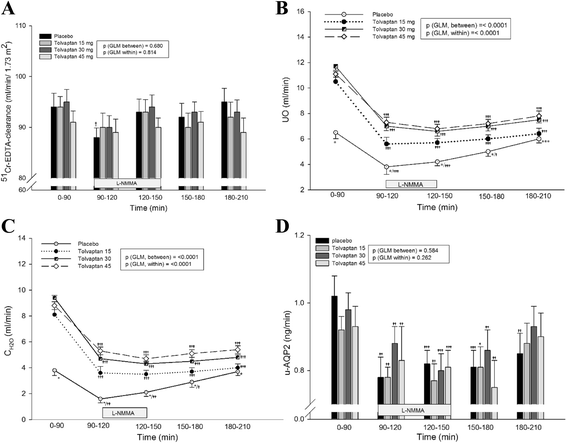
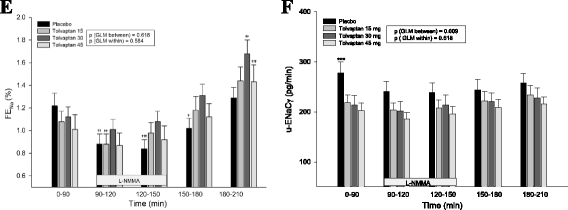
Results: During baseline, FENa was unchanged. Tolvaptan decreased u-ENaCγ dose-dependently and increased p-AVP threefold, whereas u-AQP2 was unchanged. During tolvaptan with NO-inhibition, UO and CH2O decreased dose-dependently. FENa decreased dose-independently and u-ENaCγ remained unchanged. Central BP increased equally after all treatments.
Conclusions: During baseline, fractional excretion of sodium was unchanged. During tolvaptan with NO-inhibition, renal water excretion was reduced dose dependently, and renal sodium excretion was reduced unrelated to the dose, partly via an AVP dependent mechanism. Thus, tolvaptan antagonized the reduction in renal water and sodium excretion during NO-inhibition. Most likely, the lack of decrease in AQP2 excretion by tolvaptan could be attributed to a counteracting effect of the high level of p-AVP.
Trial registration: Clinical Trial no: NCT02078973 . Registered 1 March 2014.
Keywords: AQP2; Blood pressure; ENaC; Nitric oxide; Tolvaptan; Vasoactive hormones.
Figures

References
-
- Nielsen S, Kwon TH, Christensen BM, Promeneur D, Frokiaer J, Marples D. Physiology and pathophysiology of renal aquaporins. J Am Soc Nephrol. 1999;10:647–63. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

