Activation of CD137 Signaling Promotes Angiogenesis in Atherosclerosis via Modulating Endothelial Smad1/5-NFATc1 Pathway
- PMID: 28288971
- PMCID: PMC5524009
- DOI: 10.1161/JAHA.116.004756
Activation of CD137 Signaling Promotes Angiogenesis in Atherosclerosis via Modulating Endothelial Smad1/5-NFATc1 Pathway
Retraction in
-
Retraction of: Activation of CD137 Signaling Promotes Angiogenesis in Atherosclerosis via Modulating Endothelial Smad1/5-NFATc1 Pathway.J Am Heart Assoc. 2022 May 3;11(9):e020802. doi: 10.1161/JAHA.116.020802. Epub 2022 Apr 12. J Am Heart Assoc. 2022. PMID: 35414186 Free PMC article. No abstract available.
Abstract
Background: Excessive angiogenesis is a key feature of vulnerable atherosclerotic plaques, and is considered an independent predictor of cardiovascular risk. CD137 signaling has previously been shown to be involved in atherosclerosis. However, the possible role of CD137 signaling in regulating angiogenesis has not been reported.
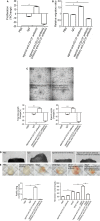
Methods and results: Apolipoprotein E-deficient (ApoE-/-) mice were used as the in vivo model of atherosclerosis. Masson and immunohistochemical analysis of atherosclerotic plaques and Matrigel plug assay were used to evaluate the angiogenesis. Human umbilical vein endothelial cells and mouse brain microvascular endothelial cells were used as in vitro and ex vivo models to study how CD137 signaling affects angiogenesis. Matrigel tube formation assay, mouse aortic ring angiogenesis assay, and migration and proliferation assay were employed to assess angiogenesis. Western blot was used to detect protein expression. We found increased neovessel formation in atherosclerotic plaques of ApoE-/- mice treated with agonist anti-CD137 antibody. Activation of CD137 signaling induced angiogenesis, endothelial proliferation, and endothelial cell migration. CD137 signaling activates the pro-angiogenic Smad1/5 pathway, induces the phosphorylation of Smad1/5 and nuclear translocation of p-Smad1/5, which in turn promotes the expression and translocation of NFATc1. Blocking CD137 signaling with inhibitory anti-CD137 antibody could inhibit this activation and attenuated agonist anti-CD137 antibody-induced angiogenesis.
Conclusions: These findings suggest that CD137 signaling is a new regulator of angiogenesis by modulating the Smad1/5-NFATc1 pathway.
Keywords: CD137; Smad1/5; angiogenesis; atherosclerosis; nuclear factor of activated T cells 1.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures





References
-
- Milei J, Parodi JC, Alonso GF, Barone A, Grana D, Matturri L. Carotid rupture and intraplaque hemorrhage: immunophenotype and role of cells involved. Am Heart J. 1998;136:1096–1105. - PubMed
-
- Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, Badimon JJ, O'Connor WN. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–2038. - PubMed
-
- Olofsson PS, Söderström LA, Wågsäter D, Sheikine Y, Ocaya P, Lang F, Rabu C, Chen L, Rudling M, Aukrust P, Hedin U, Paulsson‐Berne G, Sirsjö A, Hansson GK. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation. 2008;117:1292–1301. - PubMed
-
- Jeon HJ, Choi JH, Jung IH, Park JG, Lee MR, Lee MN, Kim B, Yoo JY, Jeong SJ, Kim DY, Park JE, Park HY, Kwack K, Choi BK, Kwon BS, Oh GT. CD137 (4‐1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation. 2010;121:1124–1133. - PubMed
-
- Yan J, Gong J, Liu P, Wang C, Chen G. Positive correlation between CD137 expression and complex stenosis morphology in patients with acute coronary syndromes. Clin Chim Acta. 2011;412:993–998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

