Prescription rates of adrenaline auto-injectors for children in UK general practice: a retrospective cohort study
- PMID: 28289013
- PMCID: PMC5565824
- DOI: 10.3399/bjgp17X689917
Prescription rates of adrenaline auto-injectors for children in UK general practice: a retrospective cohort study
Abstract
Background: Adrenaline auto-injectors (AAI) should be provided to individuals considered to be at high risk of anaphylaxis. There is some evidence that the rate of AAI prescription is increasing, but the true extent has not been previously quantified.
Aim: To estimate the trends in annual GP-issued prescriptions for AAI among UK children between 2000 and 2012.
Design and setting: Retrospective cohort study using data from primary care practices that contributed to The Health Improvement Network (THIN) database.
Method: Children and young people aged between 0-17 years of age with a prescription for AAIs were identified, and annual AAI device prescription rates were estimated using Stata (version 12).
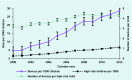
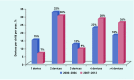
Results: A total of 1.06 million UK children were identified, providing 5.1 million person years of follow-up data. Overall, 23 837 children were deemed high risk by their GPs, and were prescribed 98 737 AAI devices. This equates to 4.67 children (95% confidence interval [CI] = 4.66 to 4.69), and 19.4 (95% CI = 19.2 to 19.5) devices per 1000 person years. Between 2000 and 2012, there has been a 355% increase in the number of children prescribed devices, and a 506% increase in the total number of AAI devices prescribed per 1000 person years in the UK. The number of devices issued per high-risk child during this period has also increased by 33%.
Conclusion: The number of children being prescribed AAI devices and the number of devices being prescribed in UK primary care between 2000 and 2012 has significantly increased. A discussion to promote rational prescribing of AAIs in the NHS is needed.
Keywords: adrenaline; allergy; anaphylaxis; database; general practice; prescriptions; primary health care.
© British Journal of General Practice 2017.
Figures
Similar articles
-
Evaluation of adrenaline auto-injector prescription profiles: A population-based, retrospective cohort study within the National Insurance Claims Database of Japan.Allergol Int. 2022 Jul;71(3):354-361. doi: 10.1016/j.alit.2022.02.002. Epub 2022 Mar 21. Allergol Int. 2022. PMID: 35331624
-
Is There an Optimal Training Interval to Improve the Correct Use of Adrenaline Auto-Injectors?Int Arch Allergy Immunol. 2020;181(2):136-140. doi: 10.1159/000504365. Epub 2019 Dec 3. Int Arch Allergy Immunol. 2020. PMID: 31794965
-
Patterns of Carriage of Prescribed Adrenaline Autoinjectors in 10- to 14-Year-Old Food-Allergic Students: A Population-Based Study.J Allergy Clin Immunol Pract. 2019 Feb;7(2):437-443. doi: 10.1016/j.jaip.2018.06.025. Epub 2018 Jul 19. J Allergy Clin Immunol Pract. 2019. PMID: 30031901
-
What are the 'ideal' features of an adrenaline (epinephrine) auto-injector in the treatment of anaphylaxis?Allergy. 2011 Jan;66(1):15-24. doi: 10.1111/j.1398-9995.2010.02450.x. Epub 2010 Aug 17. Allergy. 2011. PMID: 20716315 Review.
-
Epinephrine auto-injector pandemic.Pediatr Emerg Care. 2012 Sep;28(9):938-42. doi: 10.1097/PEC.0b013e318267f689. Pediatr Emerg Care. 2012. PMID: 22940899 Review.
Cited by
-
Accidental adrenaline auto-injector-induced digital ischaemia: a proposed treatment algorithm.BMJ Case Rep. 2021 Apr 28;14(4):e237016. doi: 10.1136/bcr-2020-237016. BMJ Case Rep. 2021. PMID: 33910787 Free PMC article.
-
EAACI Task force Clinical epidemiology of anaphylaxis: experts' perspective on the use of adrenaline autoinjectors in Europe.Clin Transl Allergy. 2020 May 11;10:12. doi: 10.1186/s13601-020-00317-y. eCollection 2020. Clin Transl Allergy. 2020. PMID: 32426107 Free PMC article.
-
Adrenaline Auto-Injectors for Preventing Fatal Anaphylaxis.Clin Exp Allergy. 2025 Jan;55(1):19-35. doi: 10.1111/cea.14565. Epub 2024 Oct 9. Clin Exp Allergy. 2025. PMID: 39383344 Free PMC article. Review.
-
Anaphylaxis triggers in a large tertiary care hospital in Qatar: a retrospective study.World Allergy Organ J. 2018 Sep 4;11(1):20. doi: 10.1186/s40413-018-0200-9. eCollection 2018. World Allergy Organ J. 2018. PMID: 30214658 Free PMC article.
-
A national survey of Russian physicians' knowledge of diagnosis and management of food-induced anaphylaxis.BMJ Open. 2017 Jul 20;7(7):e015901. doi: 10.1136/bmjopen-2017-015901. BMJ Open. 2017. PMID: 28729318 Free PMC article.
References
-
- Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report — Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47(4):373–380. - PubMed
-
- Medicines and Health Products Regulatory Agency Adrenaline auto-injectors: a review of clinical and quality considerations. 2014. http://www.mhra.gov.uk/home/groups/comms-ic/documents/websiteresources/c... (accessed 16 Feb 2017).
-
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69(8):1026–1045. - PubMed
-
- Clark A, Lloyd K, Sheikh A, et al. The RCPCH care pathway for children at risk of anaphylaxis: an evidence and consensus based national approach to caring for children with life-threatening allergies. Arch Dis Child. 2011;96(Suppl 2):i6–i9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical