Age, Weight, and CYP2D6 Genotype Are Major Determinants of Primaquine Pharmacokinetics in African Children
- PMID: 28289025
- PMCID: PMC5404566
- DOI: 10.1128/AAC.02590-16
Age, Weight, and CYP2D6 Genotype Are Major Determinants of Primaquine Pharmacokinetics in African Children
Abstract
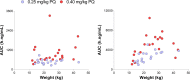
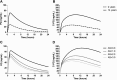
Low-dose primaquine is recommended to prevent Plasmodium falciparum malaria transmission in areas threatened by artemisinin resistance and areas aiming for malaria elimination. Community treatment campaigns with artemisinin-based combination therapy in combination with the gametocytocidal primaquine dose target all age groups, but no studies thus far have assessed the pharmacokinetics of this gametocytocidal drug in African children. We recruited 40 children participating in a primaquine efficacy trial in Burkina Faso to study primaquine pharmacokinetics. These children received artemether-lumefantrine and either a 0.25- or a 0.40-mg/kg primaquine dose. Seven blood samples were collected from each participant for primaquine and carboxy-primaquine plasma levels determinations: one sample was collected before primaquine administration and six after primaquine administration according to partially overlapping sampling schedules. Physiological population pharmacokinetic modeling was used to assess the impact of weight, age, and CYP2D6 genotype on primaquine and carboxy-primaquine pharmacokinetics. Despite linear weight normalized dosing, the areas under the plasma concentration-time curves and the peak concentrations for both primaquine and carboxy-primaquine increased with age and body weight. Children who were CYP2D6 poor metabolizers had higher levels of the parent compound, indicating a lower primaquine CYP2D6-mediated metabolism. Our data indicate that primaquine and carboxy-primaquine pharmacokinetics are influenced by age, weight, and CYP2D6 genotype and suggest that dosing strategies may have to be reconsidered to maximize the transmission-blocking properties of primaquine. (This study has been registered at ClinicalTrials.gov under registration no. NCT01935882.).
Keywords: CYP2D6; Plasmodium falciparum; pharmacokinetics; primaquine.
Copyright © 2017 Gonçalves et al.
Figures



References
-
- World Health Organization. 2012. Single dose primaquine as a gametocytocide in Plasmodium falciparum malaria. World Health Organization, Geneva, Switzerland.
-
- Eziefula AC, Bousema T, Yeung S, Kamya M, Owaraganise A, Gabagaya G, Bradley J, Grignard L, Lanke KH, Wanzira H, Mpimbaza A, Nsobya S, White NJ, Webb EL, Staedke SG, Drakeley C. 2014. Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomised, controlled, double-blind, dose-ranging trial. Lancet Infect Dis 14:130–139. doi:10.1016/S1473-3099(13)70268-8. - DOI - PubMed
-
- Goncalves BP, Tiono AB, Ouedraogo A, Guelbeogo WM, Bradley J, Nebie I, Siaka D, Lanke K, Eziefula AC, Diarra A, Pett H, Bougouma EC, Sirima SB, Drakeley C, Bousema T. 2016. Single low dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission after artemether-lumefantrine in children with asymptomatic infection: a randomised, double-blind, placebo-controlled trial. BMC Med 14:40. doi:10.1186/s12916-016-0581-y. - DOI - PMC - PubMed
-
- Dicko A, Brown JM, Diawara H, Baber I, Mahamar A, Soumare HM, Sanogo K, Koita F, Keita S, Traore SF, Chen I, Poirot E, Hwang J, McCulloch C, Lanke K, Pett H, Niemi M, Nosten F, Bousema T, Gosling R. 2016. Primaquine to reduce transmission of Plasmodium falciparum malaria in Mali: a single-blind, dose-ranging, adaptive randomised phase 2 trial. Lancet Infect Dis 16:674–684. doi:10.1016/S1473-3099(15)00479-X. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

