Fumarate Mediates a Chronic Proliferative Signal in Fumarate Hydratase-Inactivated Cancer Cells by Increasing Transcription and Translation of Ferritin Genes
- PMID: 28289076
- PMCID: PMC5440649
- DOI: 10.1128/MCB.00079-17
Fumarate Mediates a Chronic Proliferative Signal in Fumarate Hydratase-Inactivated Cancer Cells by Increasing Transcription and Translation of Ferritin Genes
Abstract
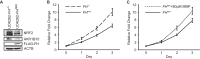
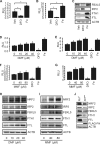
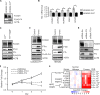
Germ line mutations of the gene encoding the tricarboxylic acid (TCA) cycle enzyme fumarate hydratase (FH) cause a hereditary cancer syndrome known as hereditary leiomyomatosis and renal cell cancer (HLRCC). HLRCC-associated tumors harbor biallelic FH inactivation that results in the accumulation of the TCA cycle metabolite fumarate. Although it is known that fumarate accumulation can alter cellular signaling, if and how fumarate confers a growth advantage remain unclear. Here we show that fumarate accumulation confers a chronic proliferative signal by disrupting cellular iron signaling. Specifically, fumarate covalently modifies cysteine residues on iron regulatory protein 2 (IRP2), rendering it unable to repress ferritin mRNA translation. Simultaneously, fumarate increases ferritin gene transcription by activating the NRF2 (nuclear factor [erythroid-derived 2]-like 2) transcription factor. In turn, increased ferritin protein levels promote the expression of the promitotic transcription factor FOXM1 (Forkhead box protein M1). Consistently, clinical HLRCC tissues showed increased expression levels of both FOXM1 and its proliferation-associated target genes. This finding demonstrates how FH inactivation can endow cells with a growth advantage.
Keywords: FH; FOXM1; HLRCC; NRF2; ferritin; fumarate.
Copyright © 2017 American Society for Microbiology.
Figures




Similar articles
-
UOK 262 cell line, fumarate hydratase deficient (FH-/FH-) hereditary leiomyomatosis renal cell carcinoma: in vitro and in vivo model of an aberrant energy metabolic pathway in human cancer.Cancer Genet Cytogenet. 2010 Jan 1;196(1):45-55. doi: 10.1016/j.cancergencyto.2009.08.018. Cancer Genet Cytogenet. 2010. PMID: 19963135 Free PMC article.
-
Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status.J Pathol. 2011 Sep;225(1):4-11. doi: 10.1002/path.2932. Epub 2011 Jun 1. J Pathol. 2011. PMID: 21630274
-
Reduced expression of fumarate hydratase in clear cell renal cancer mediates HIF-2α accumulation and promotes migration and invasion.PLoS One. 2011;6(6):e21037. doi: 10.1371/journal.pone.0021037. Epub 2011 Jun 14. PLoS One. 2011. PMID: 21695080 Free PMC article.
-
Fumarate hydratase inactivation in renal tumors: HIF1α, NRF2, and "cryptic targets" of transcription factors.Chin J Cancer. 2012 Sep;31(9):413-20. doi: 10.5732/cjc.012.10102. Epub 2012 Jul 2. Chin J Cancer. 2012. PMID: 22776233 Free PMC article. Review.
-
Fumarate hydratase as a therapeutic target in renal cancer.Expert Opin Ther Targets. 2020 Sep;24(9):923-936. doi: 10.1080/14728222.2020.1804862. Epub 2020 Oct 6. Expert Opin Ther Targets. 2020. PMID: 32744123 Review.
Cited by
-
Evaluation of disease staging and chemotherapeutic response in non-small cell lung cancer from patient tumor-derived metabolomic data.Lung Cancer. 2021 Jun;156:20-30. doi: 10.1016/j.lungcan.2021.04.012. Epub 2021 Apr 15. Lung Cancer. 2021. PMID: 33882406 Free PMC article.
-
The Tricarboxylic Acid Cycle Metabolites for Cancer: Friend or Enemy.Research (Wash D C). 2024 Jun 12;7:0351. doi: 10.34133/research.0351. eCollection 2024. Research (Wash D C). 2024. PMID: 38867720 Free PMC article. Review.
-
Identifying new biomarkers of aggressive Group 3 and SHH medulloblastoma using 3D hydrogel models, single cell RNA sequencing and 3D OrbiSIMS imaging.Acta Neuropathol Commun. 2023 Jan 11;11(1):6. doi: 10.1186/s40478-022-01496-4. Acta Neuropathol Commun. 2023. PMID: 36631900 Free PMC article.
-
Proteogenomic landscape of uterine leiomyomas from hereditary leiomyomatosis and renal cell cancer patients.Sci Rep. 2021 Apr 30;11(1):9371. doi: 10.1038/s41598-021-88585-x. Sci Rep. 2021. PMID: 33931688 Free PMC article.
-
Perturbation of Iron Metabolism by Cisplatin through Inhibition of Iron Regulatory Protein 2.Cell Chem Biol. 2019 Jan 17;26(1):85-97.e4. doi: 10.1016/j.chembiol.2018.10.009. Epub 2018 Nov 15. Cell Chem Biol. 2019. PMID: 30449675 Free PMC article.
References
-
- Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, Roylance RR, Olpin S, Bevan S, Barker K, Hearle N, Houlston RS, Kiuru M, Lehtonen R, Karhu A, Vilkki S, Laiho P, Eklund C, Vierimaa O, Aittomaki K, Hietala M, Sistonen P, Paetau A, Salovaara R, Herva R, Launonen V, Aaltonen LA, Multiple Leiomyoma Consortium . 2002. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 30:406–410. doi:10.1038/ng849. - DOI - PubMed
-
- Ternette N, Yang M, Laroyia M, Kitagawa M, O'Flaherty L, Wolhulter K, Igarashi K, Saito K, Kato K, Fischer R, Berquand A, Kessler BM, Lappin T, Frizzell N, Soga T, Adam J, Pollard PJ. 2013. Inhibition of mitochondrial aconitase by succination in fumarate hydratase deficiency. Cell Rep 3:689–700. doi:10.1016/j.celrep.2013.02.013. - DOI - PMC - PubMed
-
- Ooi A, Wong JC, Petillo D, Roossien D, Perrier-Trudova V, Whitten D, Min BW, Tan MH, Zhang Z, Yang XJ, Zhou M, Gardie B, Molinie V, Richard S, Tan PH, Teh BT, Furge KA. 2011. An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell 20:511–523. doi:10.1016/j.ccr.2011.08.024. - DOI - PubMed
-
- Yang Y, Valera VA, Padilla-Nash HM, Sourbier C, Vocke CD, Vira MA, Abu-Asab MS, Bratslavsky G, Tsokos M, Merino MJ, Pinto PA, Srinivasan R, Ried T, Neckers L, Linehan WM. 2010. UOK 262 cell line, fumarate hydratase deficient (FH−/FH−) hereditary leiomyomatosis renal cell carcinoma: in vitro and in vivo model of an aberrant energy metabolic pathway in human cancer. Cancer Genet Cytogenet 196:45–55. doi:10.1016/j.cancergencyto.2009.08.018. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
