Contribution of type III interferons to antiviral immunity: location, location, location
- PMID: 28289095
- PMCID: PMC5418032
- DOI: 10.1074/jbc.R117.777102
Contribution of type III interferons to antiviral immunity: location, location, location
Abstract
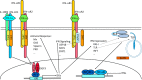
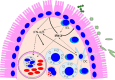
Type I interferons (IFN-α/β) and the more recently identified type III IFNs (IFN-λ) function as the first line of defense against virus infection and regulate the development of both innate and adaptive immune responses. Type III IFNs were originally identified as a novel ligand-receptor system acting in parallel with type I IFNs, but subsequent studies have provided increasing evidence for distinct roles for each IFN family. In addition to their compartmentalized antiviral actions, these two systems appear to have multiple levels of cross-regulation and act coordinately to achieve effective antimicrobial protection with minimal collateral damage to the host.
Keywords: antiviral agent; interferon; mucosal immunology; viral immunology; virology.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures

References
-
- Krause C. D., and Pestka S. (2015) Cut, copy, move, delete: the study of human interferon genes reveals multiple mechanisms underlying their evolution in amniotes. Cytokine 76, 480–495 - PubMed
-
- Qi Z., Nie P., Secombes C. J., and Zou J. (2010) Intron-containing type I and type III IFN coexist in amphibians: refuting the concept that a retroposition event gave rise to type I IFNs. J. Immunol. 184, 5038–5046 - PubMed
-
- Kotenko S. V., and Langer J. A. (2004) Full house: 12 receptors for 27 cytokines. Int. Immunopharmacol. 4, 593–608 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

