Helios expression coordinates the development of a subset of striatopallidal medium spiny neurons
- PMID: 28289129
- PMCID: PMC5399659
- DOI: 10.1242/dev.138248
Helios expression coordinates the development of a subset of striatopallidal medium spiny neurons
Abstract
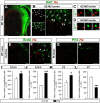
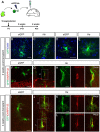
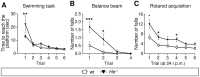
Here, we unravel the mechanism of action of the Ikaros family zinc finger protein Helios (He) during the development of striatal medium spiny neurons (MSNs). He regulates the second wave of striatal neurogenesis involved in the generation of striatopallidal neurons, which express dopamine 2 receptor and enkephalin. To exert this effect, He is expressed in neural progenitor cells (NPCs) keeping them in the G1/G0 phase of the cell cycle. Thus, a lack of He results in an increase of S-phase entry and S-phase length of NPCs, which in turn impairs striatal neurogenesis and produces an accumulation of the number of cycling NPCs in the germinal zone (GZ), which end up dying at postnatal stages. Therefore, He-/- mice show a reduction in the number of dorso-medial striatal MSNs in the adult that produces deficits in motor skills acquisition. In addition, overexpression of He in NPCs induces misexpression of DARPP-32 when transplanted in mouse striatum. These findings demonstrate that He is involved in the correct development of a subset of striatopallidal MSNs and reveal new cellular mechanisms for neuronal development.
Keywords: Cell cycle; Cell death; Ikaros; Ikzf2; Medium spiny neurons; Neurogenesis.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

