Photocyclic behavior of rhodopsin induced by an atypical isomerization mechanism
- PMID: 28289214
- PMCID: PMC5380078
- DOI: 10.1073/pnas.1617446114
Photocyclic behavior of rhodopsin induced by an atypical isomerization mechanism
Abstract
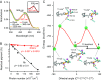
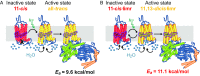
Vertebrate rhodopsin (Rh) contains 11-cis-retinal as a chromophore to convert light energy into visual signals. On absorption of light, 11-cis-retinal is isomerized to all-trans-retinal, constituting a one-way reaction that activates transducin (Gt) followed by chromophore release. Here we report that bovine Rh, regenerated instead with a six-carbon-ring retinal chromophore featuring a C11=C12 double bond locked in its cis conformation (Rh6mr), employs an atypical isomerization mechanism by converting 11-cis to an 11,13-dicis configuration for prolonged Gt activation. Time-dependent UV-vis spectroscopy, HPLC, and molecular mechanics analyses revealed an atypical thermal reisomerization of the 11,13-dicis to the 11-cis configuration on a slow timescale, which enables Rh6mr to function in a photocyclic manner similar to that of microbial Rhs. With this photocyclic behavior, Rh6mr repeatedly recruits and activates Gt in response to light stimuli, making it an excellent candidate for optogenetic tools based on retinal analog-bound vertebrate Rhs. Overall, these comprehensive structure-function studies unveil a unique photocyclic mechanism of Rh activation by an 11-cis-to-11,13-dicis isomerization.
Keywords: GPCR; chromophore; isomerization; rhodopsin; vision.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Divergent pathways in photobleaching of 7,9-dicis-rhodopsin and 9,11-dicis-12-fluororhodopsin: one-photon-two-bond and one-photon-one-bond isomerization.Biochemistry. 1993 Sep 28;32(38):10233-8. doi: 10.1021/bi00089a045. Biochemistry. 1993. PMID: 8399151
-
Molecular bases for the selection of the chromophore of animal rhodopsins.Proc Natl Acad Sci U S A. 2015 Dec 15;112(50):15297-302. doi: 10.1073/pnas.1510262112. Epub 2015 Nov 25. Proc Natl Acad Sci U S A. 2015. PMID: 26607446 Free PMC article.
-
Studies on structure and function of rhodopsin by use of cyclopentatrienylidene 11-cis-locked-rhodopsin.Biochemistry. 1984 Nov 20;23(24):5826-32. doi: 10.1021/bi00319a023. Biochemistry. 1984. PMID: 6098298
-
Photoisomerization in rhodopsin.Biochemistry (Mosc). 2001 Nov;66(11):1197-209. doi: 10.1023/a:1013123016803. Biochemistry (Mosc). 2001. PMID: 11743865 Review.
-
Mechanism of G-protein activation by rhodopsin.Photochem Photobiol. 2007 Jan-Feb;83(1):70-5. doi: 10.1562/2006-03-22-IR-854. Photochem Photobiol. 2007. PMID: 16800722 Review.
Cited by
-
A novel small molecule chaperone of rod opsin and its potential therapy for retinal degeneration.Nat Commun. 2018 May 17;9(1):1976. doi: 10.1038/s41467-018-04261-1. Nat Commun. 2018. PMID: 29773803 Free PMC article.
-
Theoretical study on the structure, spectroscopic, and current-voltage behavior of 11-Cis and Trans retinal isomers in rhodopsin.Sci Rep. 2024 May 30;14(1):12452. doi: 10.1038/s41598-024-63249-8. Sci Rep. 2024. PMID: 38816529 Free PMC article.
-
Capturing a rhodopsin receptor signalling cascade across a native membrane.Nature. 2022 Apr;604(7905):384-390. doi: 10.1038/s41586-022-04547-x. Epub 2022 Apr 6. Nature. 2022. PMID: 35388214 Free PMC article.
-
A short story on how chromophore is hydrolyzed from rhodopsin for recycling.Bioessays. 2023 Sep;45(9):e2300068. doi: 10.1002/bies.202300068. Epub 2023 Jul 16. Bioessays. 2023. PMID: 37454357 Free PMC article. Review.
-
Targeting G protein-coupled receptor signaling at the G protein level with a selective nanobody inhibitor.Nat Commun. 2018 May 18;9(1):1996. doi: 10.1038/s41467-018-04432-0. Nat Commun. 2018. PMID: 29777099 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

