Modulating IgG effector function by Fc glycan engineering
- PMID: 28289219
- PMCID: PMC5380036
- DOI: 10.1073/pnas.1702173114
Modulating IgG effector function by Fc glycan engineering
Abstract
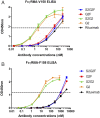
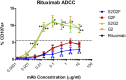
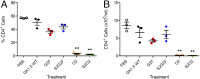
IgG antibodies contain a conserved N-glycosylation site on the Fc domain to which a complex, biantennary glycan is attached. The fine structures of this glycan modulate antibody effector functions by affecting the binding affinity of the Fc to diverse Fc receptor family members. For example, core fucosylation significantly decreases antibody-dependent cellular cytotoxicity (ADCC), whereas terminal α2,6-sialylation plays a critical role in the anti-inflammatory activity of human i.v. immunoglobulin therapy. The effect of specific combinations of sugars in the glycan on ADCC remains to be further addressed, however. Therefore, we synthesized structurally well-defined homogeneous glycoforms of antibodies with different combinations of fucosylation and sialylation and performed side-by-side in vitro FcγR-binding analyses, cell-based ADCC assays, and in vivo IgG-mediated cellular depletion studies. We found that core fucosylation exerted a significant adverse effect on FcγRIIIA binding, in vitro ADCC, and in vivo IgG-mediated cellular depletion, regardless of sialylation status. In contrast, the effect of sialylation on ADCC was dependent on the status of core fucosylation. Sialylation in the context of core fucosylation significantly decreased ADCC in a cell-based assay and suppressed antibody-mediated cell killing in vivo. In contrast, in the absence of fucosylation, sialylation did not adversely impact ADCC.
Keywords: Fc receptor; IgG; glycosylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: The anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol. 2010;30(Suppl 1):S9–S14. - PubMed
-
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. - PubMed
-
- Takahashi N, Nakagawa H, Fujikawa K, Kawamura Y, Tomiya N. Three-dimensional elution mapping of pyridylaminated N-linked neutral and sialyl oligosaccharides. Anal Biochem. 1995;226(1):139–146. - PubMed
-
- Wormald MR, et al. Variations in oligosaccharide-protein interactions in immunoglobulin G determine the site-specific glycosylation profiles and modulate the dynamic motion of the Fc oligosaccharides. Biochemistry. 1997;36(6):1370–1380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

