Gentamicin B1 is a minor gentamicin component with major nonsense mutation suppression activity
- PMID: 28289221
- PMCID: PMC5380045
- DOI: 10.1073/pnas.1620982114
Gentamicin B1 is a minor gentamicin component with major nonsense mutation suppression activity
Retraction in
-
Retraction for Baradaran-Heravi et al., Gentamicin B1 is a minor gentamicin component with major nonsense mutation suppression activity.Proc Natl Acad Sci U S A. 2018 Dec 11;115(50):E11885. doi: 10.1073/pnas.1818172115. Epub 2018 Nov 26. Proc Natl Acad Sci U S A. 2018. PMID: 30478049 Free PMC article. No abstract available.
Abstract
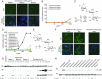
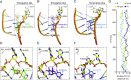
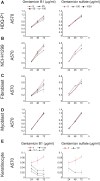
Nonsense mutations underlie about 10% of rare genetic disease cases. They introduce a premature termination codon (PTC) and prevent the formation of full-length protein. Pharmaceutical gentamicin, a mixture of several related aminoglycosides, is a frequently used antibiotic in humans that can induce PTC readthrough and suppress nonsense mutations at high concentrations. However, testing of gentamicin in clinical trials has shown that safe doses of this drug produce weak and variable readthrough activity that is insufficient for use as therapy. In this study we show that the major components of pharmaceutical gentamicin lack PTC readthrough activity but the minor component gentamicin B1 (B1) is a potent readthrough inducer. Molecular dynamics simulations reveal the importance of ring I of B1 in establishing a ribosome configuration that permits pairing of a near-cognate complex at a PTC. B1 induced readthrough at all three nonsense codons in cultured cancer cells with TP53 (tumor protein p53) mutations, in cells from patients with nonsense mutations in the TPP1 (tripeptidyl peptidase 1), DMD (dystrophin), SMARCAL1 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a-like 1), and COL7A1 (collagen type VII alpha 1 chain) genes, and in an in vivo tumor xenograft model. The B1 content of pharmaceutical gentamicin is highly variable and major gentamicins suppress the PTC readthrough activity of B1. Purified B1 provides a consistent and effective source of PTC readthrough activity to study the potential of nonsense suppression for treatment of rare genetic disorders.
Keywords: cancer; gentamicin B1; nonsense mutation; premature stop codon readthrough; rare genetic diseases.
Conflict of interest statement
Conflict of interest statement: A.B.-H. and M.R. have an ownership interest in Codon-X Therapeutics. A patent application pertaining to the results presented in the paper has been filed. The authors declare no additional competing financial interests.
Figures






References
-
- Mort M, Ivanov D, Cooper DN, Chuzhanova NA. A meta-analysis of nonsense mutations causing human genetic disease. Hum Mutat. 2008;29(8):1037–1047. - PubMed
-
- Electronic Medicines Compendium (2017) Gentamicin 10mg/ml solution for injection or infusion. Available at https://www.medicines.org.uk/emc/medicine/32933. Accessed March 3, 2017
-
- Malik V, et al. Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann Neurol. 2010;67(6):771–780. - PubMed
-
- Clancy JP, et al. Evidence that systemic gentamicin suppresses premature stop mutations in patients with cystic fibrosis. Am J Respir Crit Care Med. 2001;163(7):1683–1692. - PubMed
-
- Linde L, Kerem B. Introducing sense into nonsense in treatments of human genetic diseases. Trends Genet. 2008;24(11):552–563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

