Naturally occurring fluorescence in frogs
- PMID: 28289227
- PMCID: PMC5389305
- DOI: 10.1073/pnas.1701053114
Naturally occurring fluorescence in frogs
Abstract
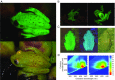
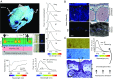
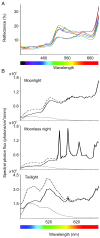
Fluorescence, the absorption of short-wavelength electromagnetic radiation reemitted at longer wavelengths, has been suggested to play several biological roles in metazoans. This phenomenon is uncommon in tetrapods, being restricted mostly to parrots and marine turtles. We report fluorescence in amphibians, in the tree frog Hypsiboas punctatus, showing that fluorescence in living frogs is produced by a combination of lymph and glandular emission, with pigmentary cell filtering in the skin. The chemical origin of fluorescence was traced to a class of fluorescent compounds derived from dihydroisoquinolinone, here named hyloins. We show that fluorescence contributes 18-29% of the total emerging light under twilight and nocturnal scenarios, largely enhancing brightness of the individuals and matching the sensitivity of night vision in amphibians. These results introduce an unprecedented source of pigmentation in amphibians and highlight the potential relevance of fluorescence in visual perception in terrestrial environments.
Keywords: Amphibia; Anura; Hylidae; fluorophore; visual ecology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lagorio MG, Cordon GB, Iriel A. Reviewing the relevance of fluorescence in biological systems. Photochem Photobiol Sci. 2015;14(9):1538–1559. - PubMed
-
- Gruber DF, Sparks JS. First observation of fluorescence in marine turtles. Am Mus Novit. 2015;3845:1–8.
-
- Stradi R, Pini E, Celentano G. The chemical structure of the pigments in Ara macao plumage. Comp Biochem Physiol B Biochem Mol Biol. 2001;130(1):57–63. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

