Control of Cellular Aging, Tissue Function, and Cancer by p53 Downstream of Telomeres
- PMID: 28289249
- PMCID: PMC5411683
- DOI: 10.1101/cshperspect.a026088
Control of Cellular Aging, Tissue Function, and Cancer by p53 Downstream of Telomeres
Abstract
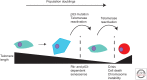
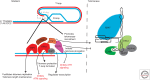
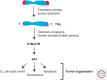
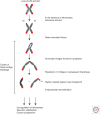
Telomeres, the nucleoprotein complex at the ends of eukaryotic chromosomes, perform an essential cellular role in part by preventing the chromosomal end from initiating a DNA-damage response. This function of telomeres can be compromised as telomeres erode either as a consequence of cell division in culture or as a normal part of cellular ageing in proliferative tissues. Telomere dysfunction in this context leads to DNA-damage signaling and activation of the tumor-suppressor protein p53, which then can prompt either cellular senescence or apoptosis. By culling cells with dysfunctional telomeres, p53 plays a critical role in protecting tissues against the effects of critically short telomeres. However, as telomere dysfunction worsens, p53 likely exacerbates short telomere-driven tissue failure diseases, including pulmonary fibrosis, aplastic anemia, and liver cirrhosis. In cells lacking p53, unchecked telomere shortening drives chromosomal end-to-end fusions and cycles of chromosome fusion-bridge-breakage. Incipient cancer cells confronting these telomere barriers must disable p53 signaling to avoid senescence and eventually up-regulate telomerase to achieve cellular immortality. The recent findings of highly recurrent activating mutations in the promoter for the telomerase reverse transcriptase (TERT) gene in diverse human cancers, together with the widespread mutations in p53 in cancer, provide support for the idea that circumvention of a telomere-p53 checkpoint is essential for malignant progression in human cancer.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
Similar articles
-
Pathways connecting telomeres and p53 in senescence, apoptosis, and cancer.Biochem Biophys Res Commun. 2005 Jun 10;331(3):881-90. doi: 10.1016/j.bbrc.2005.03.211. Biochem Biophys Res Commun. 2005. PMID: 15865944 Review.
-
Senescence and immortalization: role of telomeres and telomerase.Carcinogenesis. 2005 May;26(5):867-74. doi: 10.1093/carcin/bgh296. Epub 2004 Oct 7. Carcinogenesis. 2005. PMID: 15471900 Review.
-
Telomere dysfunction and genome instability.Front Biosci (Landmark Ed). 2012 Jun 1;17(6):2181-96. doi: 10.2741/4044. Front Biosci (Landmark Ed). 2012. PMID: 22652771 Review.
-
DNA damage response, checkpoint activation and dysfunctional telomeres: face to face between mammalian cells and Drosophila.Tsitologiia. 2013;55(4):211-7. Tsitologiia. 2013. PMID: 23875450 Review.
-
Regulation and Effect of Telomerase and Telomeric Length in Stem Cells.Curr Stem Cell Res Ther. 2021;16(7):809-823. doi: 10.2174/1574888X15666200422104423. Curr Stem Cell Res Ther. 2021. PMID: 32321410 Review.
Cited by
-
TERTp mutations and p53 expression in head and neck cutaneous basal cell carcinomas with different aggressive features.Sci Rep. 2021 May 17;11(1):10395. doi: 10.1038/s41598-021-89906-w. Sci Rep. 2021. PMID: 34001963 Free PMC article.
-
Telomeres, telomerase, and cancer: mechanisms, biomarkers, and therapeutics.Exp Hematol Oncol. 2025 Jan 27;14(1):8. doi: 10.1186/s40164-025-00597-9. Exp Hematol Oncol. 2025. PMID: 39871386 Free PMC article. Review.
-
In vitro characterization and rational analog design of a novel inhibitor of telomerase assembly in MDA MB 231 breast cancer cell line.Oncol Rep. 2022 Nov;48(5):188. doi: 10.3892/or.2022.8403. Epub 2022 Sep 14. Oncol Rep. 2022. PMID: 36102322 Free PMC article.
-
The Interactions of DNA Repair, Telomere Homeostasis, and p53 Mutational Status in Solid Cancers: Risk, Prognosis, and Prediction.Cancers (Basel). 2021 Jan 27;13(3):479. doi: 10.3390/cancers13030479. Cancers (Basel). 2021. PMID: 33513745 Free PMC article. Review.
-
Effect of Omega-3 Fatty Acids on Telomeres-Are They the Elixir of Youth?Nutrients. 2022 Sep 9;14(18):3723. doi: 10.3390/nu14183723. Nutrients. 2022. PMID: 36145097 Free PMC article. Review.
References
-
- Anderson BH, Kasher PR, Mayer J, Szynkiewicz M, Jenkinson EM, Bhaskar SS, Urquhart JE, Daly SB, Dickerson JE, O’Sullivan J, et al. 2012. Mutations in CTC1, encoding conserved telomere maintenance component 1, cause Coats plus. Nat Genet 44: 338–342. - PubMed
-
- Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, Hamosh A, et al. 2005. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci 102: 15960–15964. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
