Uncertain links in host-parasite networks: lessons for parasite transmission in a multi-host system
- PMID: 28289262
- PMCID: PMC5352821
- DOI: 10.1098/rstb.2016.0095
Uncertain links in host-parasite networks: lessons for parasite transmission in a multi-host system
Abstract
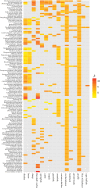
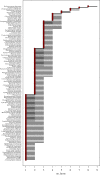
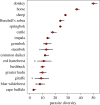
For many parasites, the full set of hosts that are susceptible to infection is not known, and this could lead to a bias in estimates of transmission. We used counts of individual adult parasites from historical parasitology studies in southern Africa to map a bipartite network of the nematode parasites of herbivore hosts that occur in Botswana. Bipartite networks are used in community ecology to represent interactions across trophic levels. We used a Bayesian hierarchical model to predict the full set of host-parasite interactions from existing data on parasitic gastrointestinal nematodes of wild and domestic ungulates given assumptions about the distribution of parasite counts within hosts, while accounting for the relative uncertainty of less sampled species. We used network metrics to assess the difference between the observed and predicted networks, and to explore the connections between hosts via their shared parasites using a host-host unipartite network projected from the bipartite network. The model predicts a large number of missing links and identifies red hartebeest, giraffe and steenbok as the hosts that have the most uncertainty in parasite diversity. Further, the unipartite network reveals clusters of herbivores that have a high degree of parasite sharing, and these clusters correspond closely with phylogenetic distance rather than with the wild/domestic boundary. These results provide a basis for predicting the risk of cross-species transmission of nematode parasites in areas where livestock and wildlife share grazing land.This article is part of the themed issue 'Opening the black box: re-examining the ecology and evolution of parasite transmission'.
Keywords: Bayesian hierarchical model; Botswana; bipartite; negative binomial; nematodes; ungulates.
© 2017 The Authors.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

