Parasite transmission in a natural multihost-multiparasite community
- PMID: 28289264
- PMCID: PMC5352823
- DOI: 10.1098/rstb.2016.0097
Parasite transmission in a natural multihost-multiparasite community
Abstract
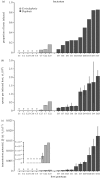
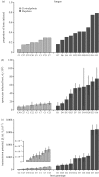
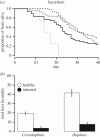
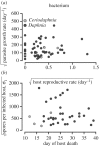
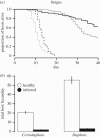
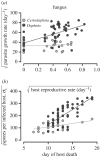
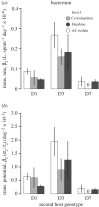
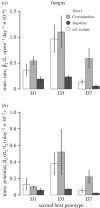
Understanding the transmission and dynamics of infectious diseases in natural communities requires understanding the extent to which the ecology, evolution and epidemiology of those diseases are shaped by alternative hosts. We performed laboratory experiments to test how parasite spillover affected traits associated with transmission in two co-occurring parasites: the bacterium Pasteuria ramosa and the fungus Metschnikowia bicuspidata Both parasites were capable of transmission from the reservoir host (Daphnia dentifera) to the spillover host (Ceriodaphnia dubia), but this occurred at a much higher rate for the fungus than the bacterium. We quantified transmission potential by combining information on parasite transmission and growth rate, and used this to compare parasite fitness in the two host species. For both parasites, transmission potential was lower in the spillover host. For the bacterium, virulence was higher in the spillover host. Transmission back to the original host was high for both parasites, with spillover influencing transmission rate of the fungus but not the bacterium. Thus, while inferior, the spillover host is not a dead-end for either parasite. Overall, our results demonstrate that the presence of multiple hosts in a community can have important consequences for disease transmission, and host and parasite fitness.This article is part of the themed issue 'Opening the black box: re-examining the ecology and evolution of parasite transmission'.
Keywords: epidemics; host–parasite interactions; spillback; spillover; virulence evolution.
© 2017 The Author(s).
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

