Hypoxia-Inducible Factor-1α Target Genes Contribute to Retinal Neuroprotection
- PMID: 28289375
- PMCID: PMC5326762
- DOI: 10.3389/fncel.2017.00020
Hypoxia-Inducible Factor-1α Target Genes Contribute to Retinal Neuroprotection
Abstract
Hypoxia-inducible factor (HIF) is a transcription factor that facilitates cellular adaptation to hypoxia and ischemia. Long-standing evidence suggests that one isotype of HIF, HIF-1α, is involved in the pathogenesis of various solid tumors and cardiac diseases. However, the role of HIF-1α in retina remains poorly understood. HIF-1α has been recognized as neuroprotective in cerebral ischemia in the past two decades. Additionally, an increasing number of studies has shown that HIF-1α and its target genes contribute to retinal neuroprotection. This review will focus on recent advances in the studies of HIF-1α and its target genes that contribute to retinal neuroprotection. A thorough understanding of the function of HIF-1α and its target genes may lead to identification of novel therapeutic targets for treating degenerative retinal diseases including glaucoma, age-related macular degeneration, diabetic retinopathy, and retinal vein occlusions.
Keywords: HIF-1α; hypoxia preconditioning; neuroprotection; retina; retinal degeneration.
Figures
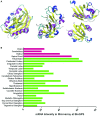
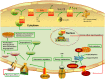
References
-
- Ando H., Natsume A., Iwami K., Ohka F., Kuchimaru T., Kizaka-Kondoh S., et al. (2013). A hypoxia-inducible factor (HIF)-3α splicing variant, HIF-3α4 impairs angiogenesis in hypervascular malignant meningiomas with epigenetically silenced HIF-3α4. Biochem. Biophys. Res. Commun. 433, 139–144. 10.1016/j.bbrc.2013.02.044 - DOI - PubMed
-
- Augstein A., Poitz D. M., Braun-Dullaeus R. C., Strasser R. H., Schmeisser A. (2011). Cell-specific and hypoxia-dependent regulation of human HIF-3α: inhibition of the expression of HIF target genes in vascular cells. Cell. Mol. Life Sci. 68, 2627–2642. 10.1007/s00018-010-0575-4 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

