Murine Cytomegalovirus Infection Induces Susceptibility to EAE in Resistant BALB/c Mice
- PMID: 28289417
- PMCID: PMC5326788
- DOI: 10.3389/fimmu.2017.00192
Murine Cytomegalovirus Infection Induces Susceptibility to EAE in Resistant BALB/c Mice
Abstract
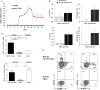
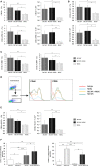
In contrast to C57BL/6 mice, BALB/c mice are relatively resistant to the induction of experimental autoimmune encephalomyelitis (EAE) after challenge with MOG35-55 peptide. Here, we provide the first evidence that infection with murine cytomegalovirus (MCMV) in adulthood abrogates this resistance. Infected BALB/c mice developed clinical and histological signs similar to those seen in susceptible C57BL/6 mice. In addition to CD4+ cells, large proportion of cells in the infiltrate of diseased BALB/c mice was CD8+, similar with findings in multiple sclerosis. CD8+ cells that responded to ex vivo restimulation with MOG35-55 were not specific for viral epitopes pp89 and m164. MCMV infection favors proinflammatory type of dendritic cells (CD86+CD40+CD11c+) in the peripheral lymph organs, M1 type of microglia in central nervous system, and increases development of Th1/Th17 encephalitogenic cells. This study indicates that MCMV may enhance autoimmune neuropathology and abrogate inherent resistance to EAE in mouse strain by enhancing proinflammatory phenotype of antigen-presenting cells, Th1/Th17, and CD8 response to MOG35-55.
Keywords: BALB/c mice; T cells; antigen-presenting cells; experimental autoimmune encephalomyelitis; murine cytomegalovirus infection.
Figures



Similar articles
-
Experimental allergic encephalomyelitis. T cell trafficking to the central nervous system in a resistant Thy-1 congenic mouse strain.Lab Invest. 1994 Nov;71(5):671-9. Lab Invest. 1994. PMID: 7526038
-
Infusion of Sulfosuccinimidyl-4-[N-maleimidomethyl]cyclohexane-1-carboxylate-Conjugated MOG35-55-Coupled Spleen Cells Effectively Prevents and Reverses Experimental Autoimmune Encephalomyelitis in Mice.J Immunol Res. 2015;2015:129682. doi: 10.1155/2015/129682. Epub 2015 Jul 14. J Immunol Res. 2015. PMID: 26258148 Free PMC article.
-
Delayed-type hypersensitivity responses to murine cytomegalovirus in genetically resistant and susceptible strains of mice.J Gen Virol. 1987 Sep;68 ( Pt 9):2379-88. doi: 10.1099/0022-1317-68-9-2379. J Gen Virol. 1987. PMID: 2821177
-
Endogenous opioid inhibition of proliferation of T and B cell subpopulations in response to immunization for experimental autoimmune encephalomyelitis.BMC Immunol. 2015 Apr 24;16:24. doi: 10.1186/s12865-015-0093-0. BMC Immunol. 2015. PMID: 25906771 Free PMC article.
-
Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis.Brain Behav Immun. 2010 May;24(4):641-51. doi: 10.1016/j.bbi.2010.01.014. Epub 2010 Feb 6. Brain Behav Immun. 2010. PMID: 20138983
Cited by
-
Interleukin-17 in Chronic Inflammatory Neurological Diseases.Front Immunol. 2020 Jun 3;11:947. doi: 10.3389/fimmu.2020.00947. eCollection 2020. Front Immunol. 2020. PMID: 32582147 Free PMC article. Review.
-
Altered characteristics of regulatory T cells in target tissues of Sjögren's syndrome in murine models.Mol Immunol. 2024 Oct;174:47-56. doi: 10.1016/j.molimm.2024.08.003. Epub 2024 Aug 27. Mol Immunol. 2024. PMID: 39197397
-
Immune responses to congenital cytomegalovirus infection.Microbes Infect. 2018 Oct-Nov;20(9-10):543-551. doi: 10.1016/j.micinf.2017.12.010. Epub 2017 Dec 26. Microbes Infect. 2018. PMID: 29287989 Free PMC article. Review.
-
Human Cytomegalovirus and Autoimmune Diseases: Where Are We?Viruses. 2021 Feb 8;13(2):260. doi: 10.3390/v13020260. Viruses. 2021. PMID: 33567734 Free PMC article. Review.
-
Delimiting MOGAD as a disease entity using translational imaging.Front Neurol. 2023 Dec 7;14:1216477. doi: 10.3389/fneur.2023.1216477. eCollection 2023. Front Neurol. 2023. PMID: 38333186 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

