Cav1.2 channels mediate persistent chronic stress-induced behavioral deficits that are associated with prefrontal cortex activation of the p25/Cdk5-glucocorticoid receptor pathway
- PMID: 28289693
- PMCID: PMC5338724
- DOI: 10.1016/j.ynstr.2017.02.004
Cav1.2 channels mediate persistent chronic stress-induced behavioral deficits that are associated with prefrontal cortex activation of the p25/Cdk5-glucocorticoid receptor pathway
Abstract
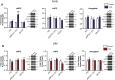
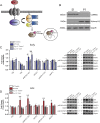
Chronic stress is known to precipitate and exacerbate neuropsychiatric symptoms, and exposure to stress is particularly pathological in individuals with certain genetic predispositions. Recent genome wide association studies have identified single nucleotide polymorphisms (SNPs) in the gene CACNA1C, which codes for the Cav1.2 subunit of the L-type calcium channel (LTCC), as a common risk variant for multiple neuropsychiatric conditions. Cav1.2 channels mediate experience-dependent changes in gene expression and long-term synaptic plasticity through activation of downstream calcium signaling pathways. Previous studies have found an association between stress and altered Cav1.2 expression in the brain, however the contribution of Cav1.2 channels to chronic stress-induced behaviors, and the precise Cav1.2 signaling mechanisms activated are currently unknown. Here we report that chronic stress leads to a delayed increase in Cav1.2 expression selectively within the prefrontal cortex (PFC), but not in other stress-sensitive brain regions such as the hippocampus or amygdala. Further, we demonstrate that while Cav1.2 heterozygous (Cav1.2+/-) mice show chronic stress-induced depressive-like behavior, anxiety-like behavior, and deficits in working memory 1-2 days following stress, they are resilient to the effects of chronic stress when tested 5-7 days later. Lastly, molecular studies find a delayed upregulation of the p25/Cdk5-glucocorticoid receptor (GR) pathway in the PFC when examined 8 days post-stress that is absent in Cav1.2+/- mice. Our findings reveal a novel Cav1.2-mediated molecular mechanism associated with the persistent behavioral effects of chronic stress and provide new insight into potential Cav1.2 channel mechanisms that may contribute to CACNA1C-linked neuropsychiatric phenotypes.
Figures





References
-
- Adzic M., Djordjevic J., Djordjevic A., Niciforovic A., Demonacos C., Radojcic M., Krstic-Demonacos M. Acute or chronic stress induce cell compartment-specific phosphorylation of glucocorticoid receptor and alter its transcriptional activity in Wistar rat brain. J. Endocrinol. 2009;202:87–97. - PMC - PubMed
-
- Ament S.A., Szelinger S., Glusman G., Ashworth J., Hou L., Akula N., Shekhtman T., Badner J.A., Brunkow M.E., Mauldin D.E., Stittrich A.B., Rouleau K., Detera-Wadleigh S.D., Nurnberger J.I., Jr., Edenberg H.J., Gershon E.S., Schork N., Bipolar Genome Study, Price ND. Gelinas R., Hood L., Craig D., McMahon F.J., Kelsoe J.R., Roach J.C. Rare variants in neuronal excitability genes influence risk for bipolar disorder. Proc. Natl. Acad. Sci. 2015;112:3576–3581. (U.S.A) - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

