Preferential TNF α signaling via TNFR2 regulates epithelial injury and duct obstruction in experimental biliary atresia
- PMID: 28289704
- PMCID: PMC5333971
- DOI: 10.1172/jci.insight.88747
Preferential TNF α signaling via TNFR2 regulates epithelial injury and duct obstruction in experimental biliary atresia
Abstract
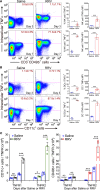
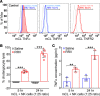
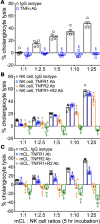
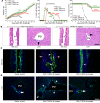
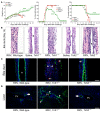
Biliary atresia is an obstructive cholangiopathy of infancy that progresses to end-stage cirrhosis. Although the pathogenesis of the disease is not completely understood, previous reports link TNFα to apoptosis of the bile duct epithelium in the presence of IFNγ. Here, we investigate if TNFα signaling regulates pathogenic mechanisms of biliary atresia. First, we quantified the expression of TNFA and its receptors TNFR1 and TNFR2 in human livers and found an increased expression of the receptors at the time of diagnosis. In mechanistic experiments using a neonatal mouse model of rhesus rotavirus-induced (RRV-induced) biliary atresia, the expression of the ligand and both receptors increased 6- to 8-fold in hepatic DCs and NK lymphocytes above controls. The activation of tissue NK cells by RRV-primed DCs was independent of TNFα-TNFR signaling. Once activated, the expression of TNFα by NK cells induced lysis of 55% ± 2% of bile duct epithelial cells, which was completely prevented by blocking TNFα or TNFR2, but not TNFR1. More notably, antibody-mediated or genetic disruption of TNFα-TNFR2 signaling in vivo decreased apoptosis and epithelial injury; suppressed the infiltration of livers by T cells, DCs, and NK cells; prevented extrahepatic bile duct obstruction; and promoted long-term survival. These findings point to a key role for the TNFα/TNFR2 axis on pathogenesis of experimental biliary atresia and identify new therapeutic targets to suppress the disease phenotype.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

