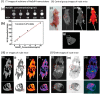
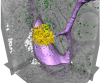
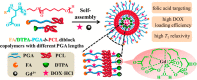
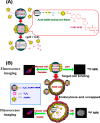
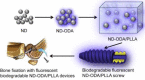
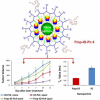
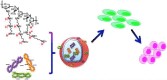
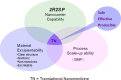
Diverse Applications of Nanomedicine
- PMID: 28290206
- PMCID: PMC5371978
- DOI: 10.1021/acsnano.6b06040
Diverse Applications of Nanomedicine
Abstract
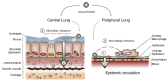
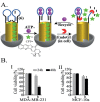
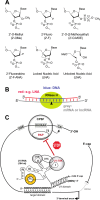
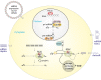
The design and use of materials in the nanoscale size range for addressing medical and health-related issues continues to receive increasing interest. Research in nanomedicine spans a multitude of areas, including drug delivery, vaccine development, antibacterial, diagnosis and imaging tools, wearable devices, implants, high-throughput screening platforms, etc. using biological, nonbiological, biomimetic, or hybrid materials. Many of these developments are starting to be translated into viable clinical products. Here, we provide an overview of recent developments in nanomedicine and highlight the current challenges and upcoming opportunities for the field and translation to the clinic.
Conflict of interest statement
The authors declare no competing financial interest.
Figures