Structural Basis of Eco1-Mediated Cohesin Acetylation
- PMID: 28290497
- PMCID: PMC5349539
- DOI: 10.1038/srep44313
Structural Basis of Eco1-Mediated Cohesin Acetylation
Erratum in
-
Erratum: Structural Basis of Eco1-Mediated Cohesin Acetylation.Sci Rep. 2017 Apr 11;7:46382. doi: 10.1038/srep46382. Sci Rep. 2017. PMID: 28397800 Free PMC article. No abstract available.
Abstract
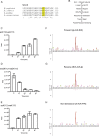
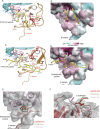
Sister-chromatid cohesion is established by Eco1-mediated acetylation on two conserved tandem lysines in the cohesin Smc3 subunit. However, the molecular basis of Eco1 substrate recognition and acetylation in cohesion is not fully understood. Here, we discover and rationalize the substrate specificity of Eco1 using mass spectrometry coupled with in-vitro acetylation assays and crystallography. Our structures of the X. laevis Eco2 (xEco2) bound to its primary and secondary Smc3 substrates demonstrate the plasticity of the substrate-binding site, which confers substrate specificity by concerted conformational changes of the central β hairpin and the C-terminal extension.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Michaelis C., Ciosk R. & Nasmyth K. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell 91, 35–45 (1997). - PubMed
-
- Ciosk R. et al.. Cohesin’s binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Mol Cell 5, 243–254 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

