In situ self-assembly of gold nanoparticles on hydrophilic and hydrophobic substrates for influenza virus-sensing platform
- PMID: 28290527
- PMCID: PMC5349514
- DOI: 10.1038/srep44495
In situ self-assembly of gold nanoparticles on hydrophilic and hydrophobic substrates for influenza virus-sensing platform
Abstract
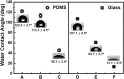
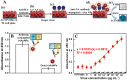
Nanomaterials without chemical linkers or physical interactions that reside on a two-dimensional surface are attractive because of their electronic, optical and catalytic properties. An in situ method has been developed to fabricate gold nanoparticle (Au NP) films on different substrates, regardless of whether they are hydrophilic or hydrophobic surfaces, including glass, 96-well polystyrene plates, and polydimethylsiloxane (PDMS). A mixture of sodium formate (HCOONa) and chloroauric acid (HAuCl4) solution was used to prepare Au NP films at room temperature. An experimental study of the mechanism revealed that film formation is dependent on surface wettability and inter particle attraction. The as-fabricated Au NP films were further applied to the colorimetric detection of influenza virus. The response to the commercial target, New Caledonia/H1N1/1999 influenza virus, was linear in the range from 10 pg/ml to 10 μg/ml and limit of detection was 50.5 pg/ml. In the presence of clinically isolated influenza A virus (H3N2), the optical density of developed color was dependent on the virus concentration (10-50,000 PFU/ml). The limit of detection of this study was 24.3 PFU/ml, a limit 116 times lower than that of conventional ELISA (2824.3 PFU/ml). The sensitivity was also 500 times greater than that of commercial immunochromatography kits.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Ahmed et al.. Metal enhanced fluorescence on nanoporous gold leaf-based assay platform for virus detection. Biosens Bioelectron 58, 33–39 (2014). - PubMed
-
- Chuan F. G., Sun T., Cao F., Liu Q. & Ren Z. Metallic nanostructures for light trapping in energy-harvesting devices. Light Sci Appl 3, e161 (2014).
-
- Atwater H. A. & Polman A. Plasmonics for improved photovoltaic devices. Nat Mater 9, 205–213 (2010). - PubMed
-
- Green M. A., Emery K., Hishikawa Y., Warta W. & Dunlop E. D. Solar cell efficiency tables (version 39). Prog Photovolt: Res Appl 20, 12–20 (2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

