Review of interferometric spectroscopy of scattered light for the quantification of subdiffractional structure of biomaterials
- PMID: 28290596
- PMCID: PMC5348632
- DOI: 10.1117/1.JBO.22.3.030901
Review of interferometric spectroscopy of scattered light for the quantification of subdiffractional structure of biomaterials
Abstract
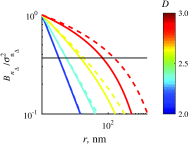
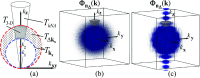
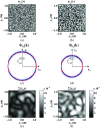
Optical microscopy is the staple technique in the examination of microscale material structure in basic science and applied research. Of particular importance to biology and medical research is the visualization and analysis of the weakly scattering biological cells and tissues. However, the resolution of optical microscopy is limited to ? 200 ?? nm due to the fundamental diffraction limit of light. We review one distinct form of the spectroscopic microscopy (SM) method, which is founded in the analysis of the second-order spectral statistic of a wavelength-dependent bright-field far-zone reflected-light microscope image. This technique offers clear advantages for biomedical research by alleviating two notorious challenges of the optical evaluation of biomaterials: the diffraction limit of light and the lack of sensitivity to biological, optically transparent structures. Addressing the first issue, it has been shown that the spectroscopic content of a bright-field microscope image quantifies structural composition of samples at arbitrarily small length scales, limited by the signal-to-noise ratio of the detector, without necessarily resolving them. Addressing the second issue, SM utilizes a reference arm, sample arm interference scheme, which allows us to elevate the weak scattering signal from biomaterials above the instrument noise floor.
Figures
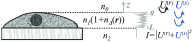

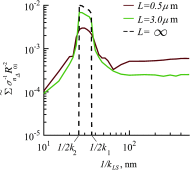

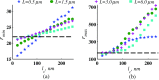
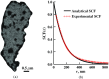

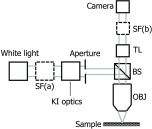

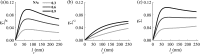
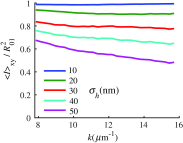
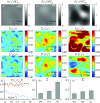
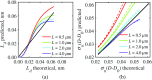
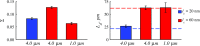
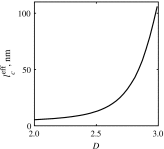
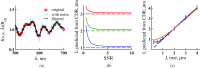
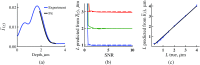

References
-
- Alexandrov S., Sampson D., “Spatial information transmission beyond a system’s diffraction limit using optical spectral encoding of the spatial frequency,” J. Opt. A: Pure Appl. Opt. 10(2), 025304 (2008). 10.1088/1464-4258/10/2/025304 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

