SMARCB1 (INI-1)-deficient Sinonasal Carcinoma: A Series of 39 Cases Expanding the Morphologic and Clinicopathologic Spectrum of a Recently Described Entity
- PMID: 28291122
- PMCID: PMC5354087
- DOI: 10.1097/PAS.0000000000000797
SMARCB1 (INI-1)-deficient Sinonasal Carcinoma: A Series of 39 Cases Expanding the Morphologic and Clinicopathologic Spectrum of a Recently Described Entity
Abstract
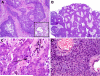
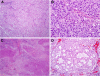
To more fully characterize the clinical and pathologic spectrum of a recently described tumor entity of the sinonasal tract characterized by loss of nuclear expression of SMARCB1 (INI1), we analyzed 39 SMARCB1-deficient sinonasal carcinomas collected from multiple medical centers. The tumors affected 23 males and 16 females with an age range of 19 to 89 years (median, 52). All patients presented with locally advanced disease (T3, n=5; T4, n=27) involving the sinuses (mainly ethmoid) with variable involvement of the nasal cavity. Thirty patients received surgery and/or radiochemotherapy with curative intent. At last follow-up, 56% of patients died of disease 0 to 102 months after diagnosis (median, 15), 2 were alive with disease, and 1 died of an unrelated cause. Only 9 patients (30%) were alive without disease at last follow-up (range, 11 to 115 mo; median, 26). The original diagnosis of retrospectively identified cases was most often sinonasal undifferentiated carcinoma (n=14) and nonkeratinizing/basaloid squamous cell carcinoma (n=5). Histologically, most tumors displayed either a predominantly basaloid (61%) or plasmacytoid/rhabdoid morphology (36%). The plasmacytoid/rhabdoid form consisted of sheets of tumor cells with abundant, eccentrically placed eosinophilic cytoplasm, whereas similar cells were typically rare and singly distributed in the basaloid variant. Glandular differentiation was seen in a few tumors. None of the cases showed squamous differentiation or surface dysplasia. By immunohistochemistry, the tumors were positive for pancytokeratin (97%), CK5 (64%), p63 (55%), and CK7 (48%); and they were negative for NUT (0%). Epstein-Barr virus and high-risk human papillomavirus was not detected by in situ hybridization. Immunohistochemical loss of SMARCB1 (INI1) expression was confirmed for all 39 tumors. Investigation of other proteins in the SWI/SNF complex revealed co-loss of SMARCA2 in 4 cases, but none were SMARCA4 deficient or ARID1A deficient. Of 27 tumors with SMARCB1 fluorescence in situ hybridization analysis, 14 showed homozygous (biallelic) deletions and 7 showed heterozygous (monoallelic) deletions. SMARCB1-deficient sinonasal carcinoma represents an emerging poorly differentiated/undifferentiated sinonasal carcinoma that (1) cannot be better classified as another specific tumor type, (2) has consistent histopathologic findings (albeit with some variability) with varying proportions of plasmacytoid/rhabdoid cells, and (3) demonstrates an aggressive clinical course. This entity should be considered in any difficult-to-classify sinonasal carcinoma, as correct diagnosis will be mandatory for optimizing therapy and for further delineation of this likely underdiagnosed disease.
Figures






References
-
- Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877–85. - PubMed
-
- Haerle SK, Gullane PJ, Witterick IJ, et al. Sinonasal carcinomas: epidemiology, pathology, and management. Neurosurg Clin N Am. 2013;24:39–49. - PubMed
-
- Mills SE, Fechner RE. “Undifferentiated” neoplasms of the sinonasal region: differential diagnosis based on clinical, light microscopic, immunohistochemical, and ultrastructural features. Semin Diagn Pathol. 1989;6:316–28. - PubMed
-
- Stelow EB, Jo VY, Mills SE, et al. A histologic and immunohistochemical study describing the diversity of tumors classified as sinonasal high-grade nonintestinal adenocarcinomas. Am J Surg Pathol. 2011;35:971–80. - PubMed
-
- Frierson HF, Jr, Mills SE, Fechner RE, et al. Sinonasal undifferentiated carcinoma. An aggressive neoplasm derived from schneiderian epithelium and distinct from olfactory neuroblastoma. Am J Surg Pathol. 1986;10:771–9. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

