Neuronal ELAVL proteins utilize AUF-1 as a co-partner to induce neuron-specific alternative splicing of APP
- PMID: 28291226
- PMCID: PMC5349543
- DOI: 10.1038/srep44507
Neuronal ELAVL proteins utilize AUF-1 as a co-partner to induce neuron-specific alternative splicing of APP
Abstract
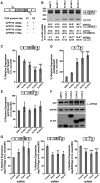
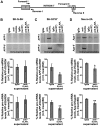
Aβ peptide that accumulates in Alzheimer's disease brain, derives from proteolytic processing of the amyloid precursor protein (APP) that exists in three main isoforms derived by alternative splicing. The isoform APP695, lacking exons 7 and 8, is predominately expressed in neurons and abnormal neuronal splicing of APP has been observed in the brain of patients with Alzheimer's disease. Herein, we demonstrate that expression of the neuronal members of the ELAVL protein family (nELAVLs) correlate with APP695 levels in vitro and in vivo. Moreover, we provide evidence that nELAVLs regulate the production of APP695; by using a series of reporters we show that concurrent binding of nELAVLs to sequences located both upstream and downstream of exon 7 is required for its skipping, whereas nELAVL-binding to a highly conserved U-rich sequence upstream of exon 8, is sufficient for its exclusion. Finally, we report that nELAVLs block APP exon 7 or 8 definition by reducing the binding of the essential splicing factor U2AF65, an effect facilitated by the concurrent binding of AUF-1. Our study provides new insights into the regulation of APP pre-mRNA processing, supports the role for nELAVLs as neuron-specific splicing regulators and reveals a novel function of AUF1 in alternative splicing.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






Similar articles
-
A role for SC35 and hnRNPA1 in the determination of amyloid precursor protein isoforms.Mol Psychiatry. 2007 Jul;12(7):681-90. doi: 10.1038/sj.mp.4001971. Epub 2007 Mar 13. Mol Psychiatry. 2007. PMID: 17353911 Free PMC article.
-
Hu antigen R (HuR) functions as an alternative pre-mRNA splicing regulator of Fas apoptosis-promoting receptor on exon definition.J Biol Chem. 2008 Jul 4;283(27):19077-84. doi: 10.1074/jbc.M800017200. Epub 2008 May 7. J Biol Chem. 2008. PMID: 18463097
-
Alternative splicing regulation of APP exon 7 by RBFox proteins.Neurochem Int. 2014 Dec;78:7-17. doi: 10.1016/j.neuint.2014.08.001. Epub 2014 Aug 11. Neurochem Int. 2014. PMID: 25125370
-
Post-transcriptional control of gene expression by AUF1: mechanisms, physiological targets, and regulation.Biochim Biophys Acta. 2013 Jun-Jul;1829(6-7):680-8. doi: 10.1016/j.bbagrm.2012.12.002. Epub 2012 Dec 14. Biochim Biophys Acta. 2013. PMID: 23246978 Free PMC article. Review.
-
Regulation and expression of the Alzheimer's beta/A4 amyloid protein precursor in health, disease, and Down's syndrome.Ann N Y Acad Sci. 1993 Sep 24;695:91-102. doi: 10.1111/j.1749-6632.1993.tb23035.x. Ann N Y Acad Sci. 1993. PMID: 8239320 Review.
Cited by
-
RNA-binding protein ELAVL4/HuD ameliorates Alzheimer's disease-related molecular changes in human iPSC-derived neurons.Prog Neurobiol. 2022 Oct;217:102316. doi: 10.1016/j.pneurobio.2022.102316. Epub 2022 Jul 14. Prog Neurobiol. 2022. PMID: 35843356 Free PMC article.
-
Unraveling the Pathways to Neuronal Homeostasis and Disease: Mechanistic Insights into the Role of RNA-Binding Proteins and Associated Factors.Int J Mol Sci. 2018 Aug 3;19(8):2280. doi: 10.3390/ijms19082280. Int J Mol Sci. 2018. PMID: 30081499 Free PMC article. Review.
-
Longitudinal scRNA-seq analysis in mouse and human informs optimization of rapid mouse astrocyte differentiation protocols.Nat Neurosci. 2023 Oct;26(10):1726-1738. doi: 10.1038/s41593-023-01424-2. Epub 2023 Sep 11. Nat Neurosci. 2023. PMID: 37697111 Free PMC article.
-
Comprehensive analysis of dysregulated circular RNAs and construction of a ceRNA network involved in the pathology of Alzheimer's disease in a 5 × FAD mouse model.Front Aging Neurosci. 2022 Nov 17;14:1020699. doi: 10.3389/fnagi.2022.1020699. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36466608 Free PMC article.
-
ELAVL2 loss promotes aggressive mesenchymal transition in glioblastoma.NPJ Precis Oncol. 2024 Mar 28;8(1):79. doi: 10.1038/s41698-024-00566-1. NPJ Precis Oncol. 2024. PMID: 38548861 Free PMC article.
References
-
- Selkoe D. J. Alzheimer’s disease: genes, proteins and therapy. Physiol Rev. 81, 741–766 (2001). - PubMed
-
- Brouwers N., Sleegers K. & Van Broeckhoven C. Molecular genetics of Alzheimer’s disease: an update. Ann Med. 40, 562–583 (2008). - PubMed
-
- Tanzi R. E. et al.. Amyloid beta protein gene: cDNA, mRNA distribution and genetic linkage near the Alzheimer locus. Science, 235, 880–884 (1987). - PubMed
-
- Schmechel D. E. et al.. Cellular localization of messenger RNA encoding amyloid-beta-protein in normal tissue and in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2, 96–111 (1988). - PubMed
-
- König G. et al.. Identification and differential expression of a novel alternative splice isoform of the beta A4 amyloid precursor protein (APP) mRNA in leukocytes and brain microglial cells. J. Biol. Chem. 267, 10804–10809 (1992). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

