Enantioselective Iridium-Catalyzed Allylic Alkylation Reactions of Masked Acyl Cyanide Equivalents
- PMID: 28291366
- PMCID: PMC5470644
- DOI: 10.1021/acs.orglett.7b00449
Enantioselective Iridium-Catalyzed Allylic Alkylation Reactions of Masked Acyl Cyanide Equivalents
Abstract
The first enantioselective iridium-catalyzed allylic alkylation reaction of a masked acyl cyanide (MAC) reagent has been developed. The transformation allows for the use of an umpoled synthon, which serves as a carbon monoxide equivalent. The reaction proceeds with good yield and excellent selectivity up to gram scale for a wide range of substituted allylic electrophiles, delivering products amenable to the synthesis of highly desirable, enantioenriched vinylated α-aryl carbonyl derivatives.
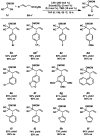
Figures
References
-
- Janssen JP, Helmchen G. Tetrahedron Lett. 1997;38:8025–8026.
-
-
For selected reviews of asymmetric iridium-catalyzed allylic alkylation, see: Helmchen G, Dahnz A, Dubon P, Schelwies M, Weihofen R. Chem Commun. 2007:675–691.Hartwig JF, Pouy MJ. Top Organomet Chem. 2011;34:169–208.Liu WB, Xia JB, You SL. Top Organomet Chem. 2011;38:155–208.Hethcox JC, Shockley SE, Stoltz BM. ACS Catal. 2016;6:6207–6213.
-
-
-
Nucleophilic additions of methylene imines to iridium π-allyl complexes have been accomplished via an umpoled strategy. However, the products delivered resemble products of standard iridium-catalyzed allylic alkylation of enolates; see: Su YL, Li YH, Chen YG, Han ZY. Chem Commun. 2017;53:1985–1988.
-
-
- Förster S, Tverskoy O, Helmchen G. Synlett. 2008;2008:2803–2806.
-
- Breitler S, Carreira EM. J Am Chem Soc. 2015;137:5296–5299. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources