Juvenile idiopathic arthritis: what is the utility of ultrasound?
- PMID: 28291375
- PMCID: PMC5605112
- DOI: 10.1259/bjr.20160920
Juvenile idiopathic arthritis: what is the utility of ultrasound?
Abstract
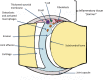
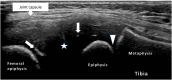
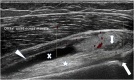
Juvenile idiopathic arthritis (JIA) is a heterogeneous condition and an important cause of acquired disability in children. Evidence supports early treatment to prevent future complications. This relies on prompt diagnosis, achieved by a high index of clinical suspicion and supportive evidence, including the detection of joint and or tendon inflammation. Ultrasound is a readily accessible, well-tolerated, safe and accurate modality for assessing joints and the surrounding soft tissues. It can also be used to guide therapy into those joints and tendon sheaths resistant to systemic treatments. Ultrasound imaging is highly operator dependent, and the developing skeleton poses unique challenges in interpretation with sonographic findings that can mimic pathology and vice versa. Ultrasound technology has been rapidly improving and is more accessible than ever before. In this article, we review the normal appearances, highlight potential pitfalls and present the key pathological findings commonly seen in JIA.
Figures
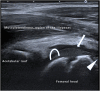
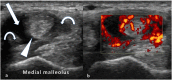
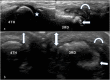
References
-
- Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine 2014; 81: 112–17. doi: https://doi.org/10.1016/j.jbspin.2013.09.003 - DOI - PubMed
-
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, He X, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001; 31. - PubMed
-
- Rigante D, Bosco A, Esposito S. The etiology of juvenile idiopathic arthritis. Clin Rev Allergy Immunol 2015; 49: 253–61. doi: https://doi.org/10.1007/s12016-014-8460-9 - DOI - PubMed
-
- Martini A. Are the number of joints involved or the presence of psoriasis still useful tools to identify homogeneous disease entities in juvenile idiopathic arthritis? J Rheumatol 2003; 30: 1900–3. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

