Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels
- PMID: 28291388
- PMCID: PMC5455597
- DOI: 10.1200/JCO.2016.71.3024
Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels
Abstract
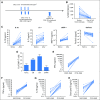
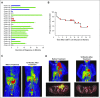
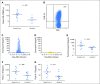
Purpose T cells genetically modified to express chimeric antigen receptors (CARs) targeting CD19 (CAR-19) have potent activity against acute lymphoblastic leukemia, but fewer results supporting treatment of lymphoma with CAR-19 T cells have been published. Patients with lymphoma that is chemotherapy refractory or relapsed after autologous stem-cell transplantation have a grim prognosis, and new treatments for these patients are clearly needed. Chemotherapy administered before adoptive T-cell transfer has been shown to enhance the antimalignancy activity of adoptively transferred T cells. Patients and Methods We treated 22 patients with advanced-stage lymphoma in a clinical trial of CAR-19 T cells preceded by low-dose chemotherapy. Nineteen patients had diffuse large B-cell lymphoma, two patients had follicular lymphoma, and one patient had mantle cell lymphoma. Patients received a single dose of CAR-19 T cells 2 days after a low-dose chemotherapy conditioning regimen of cyclophosphamide plus fludarabine. Results The overall remission rate was 73% with 55% complete remissions and 18% partial remissions. Eleven of 12 complete remissions are ongoing. Fifty-five percent of patients had grade 3 or 4 neurologic toxicities that completely resolved. The low-dose chemotherapy conditioning regimen depleted blood lymphocytes and increased serum interleukin-15 (IL-15). Patients who achieved a remission had a median peak blood CAR+ cell level of 98/μL and those who did not achieve a remission had a median peak blood CAR+ cell level of 15/μL ( P = .027). High serum IL-15 levels were associated with high peak blood CAR+ cell levels ( P = .001) and remissions of lymphoma ( P < .001). Conclusion CAR-19 T cells preceded by low-dose chemotherapy induced remission of advanced-stage lymphoma, and high serum IL-15 levels were associated with the effectiveness of this treatment regimen. CAR-19 T cells will likely become an important treatment for patients with relapsed lymphoma.
Figures

Comment in
-
Immunotherapy: DLBCL remissions driven by CARs.Nat Rev Clin Oncol. 2017 May;14(5):264. doi: 10.1038/nrclinonc.2017.48. Epub 2017 Mar 29. Nat Rev Clin Oncol. 2017. PMID: 28352132 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

