Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer
- PMID: 28291390
- PMCID: PMC5559902
- DOI: 10.1200/JCO.2016.70.8297
Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer
Abstract
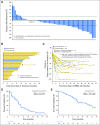
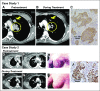
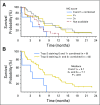
Purpose Trop-2, expressed in most triple-negative breast cancers (TNBCs), may be a potential target for antibody-drug conjugates. Sacituzumab govitecan, an antibody-drug conjugate, targets Trop-2 for the selective delivery of SN-38, the active metabolite of irinotecan. Patients and Methods We evaluated sacituzumab govitecan in a single-arm, multicenter trial in patients with relapsed/refractory metastatic TNBC who received a 10 mg/kg starting dose on days 1 and 8 of 21-day repeated cycles. The primary end points were safety and objective response rate; secondary end points were progression-free survival and overall survival. Results In 69 patients who received a median of five prior therapies (range, one to 12) since diagnosis, the confirmed objective response rate was 30% (partial response, n = 19; complete response, n = 2), the median response duration was 8.9 (95% CI, 6.1 to 11.3) months, and the clinical benefit rate (complete response + partial response + stable disease ≥ 6 months) was 46%. These responses occurred early, with a median onset of 1.9 months. Median progression-free survival was 6.0 (95% CI, 5.0 to 7.3) months, and median overall survival was 16.6 (95% CI, 11.1 to 20.6) months. Grade ≥ 3 adverse events included neutropenia (39%), leukopenia (16%), anemia (14%), and diarrhea (13%); the incidence of febrile neutropenia was 7%. The majority of archival tumor specimens (88%) were moderately to strongly positive for Trop-2 by immunohistochemistry. No neutralizing antibodies to the ADC or antibody were detected, despite repeated cycles developed. Conclusion Sacituzumab govitecan was well tolerated and induced early and durable responses in heavily pretreated patients with metastatic TNBC. As a therapeutic target and predictive biomarker, Trop-2 warrants further research.
Figures

References
-
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. - PubMed
-
- Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. - PubMed
-
- Hurvitz S, Mead M. Triple-negative breast cancer: Advancements in characterization and treatment approach. Curr Opin Obstet Gynecol. 2016;28:59–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

