Pegylated interferon alfa-2a in patients with essential thrombocythaemia or polycythaemia vera: a post-hoc, median 83 month follow-up of an open-label, phase 2 trial
- PMID: 28291640
- PMCID: PMC5421384
- DOI: 10.1016/S2352-3026(17)30030-3
Pegylated interferon alfa-2a in patients with essential thrombocythaemia or polycythaemia vera: a post-hoc, median 83 month follow-up of an open-label, phase 2 trial
Erratum in
-
Correction to Lancet Haematol 2017; 4: e165-75.Lancet Haematol. 2017 Jun;4(6):e257. doi: 10.1016/S2352-3026(17)30085-6. Lancet Haematol. 2017. PMID: 28583288 No abstract available.
Abstract
Background: Pegylated interferon alfa-2a is an immunomodulatory agent used to treat polycythemia vera. The durability of responses and long-term safety of this drug in patients with polycythaemia vera and essential thrombocythaemia have not been reported. Here, we present long-term efficacy and safety data from a single-centre, open-label, phase 2 trial, after a median of 83 months follow up.
Methods: Patients older than 18 years who were diagnosed with essential thrombocythaemia or polycythaemia vera according to 2001 WHO criteria were eligible to enrol in our study. The initial starting dose of pegylated interferon alfa-2a was 450 μg subcutaneously once per week, but was decreased in a stepwise manner due to toxic effects to a final starting dose of 90 mg per week: three patients were started at a dose of 450 mg per week, three at 360 mg per week, 19 at 270 mg per week, 26 at 180 mg per week, and 32 at 90 mg per week. Treatment was continued for as long as the patients derived clinical benefit with reductions in dose and frequency of administration allowed at the discretion of the treating physician. Haematological responses were assessed every 3-6 months on the basis of blood counts as defined by the European LeukemiaNet critieria. The primary endpoint of the initial study was the proportion of patients with a haematological response. Complete haematological response was defined as normalisation of blood counts (for patients with essential thrombocythaemia, platelets ≤440 × 109 per L; for patients with polycythaemia vera, haemoglobin <15·0 g/L without phlebotomy) with complete resolution of palpable splenomegaly or symptoms in the absence of a thrombotic event. Data were analysed with descriptive statistics and in the intention-to-treat population. This study is registered with ClinicalTrials.gov, number NCT00452023 and is ongoing but not enrolling new patients.
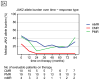
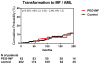
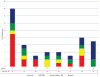
Findings: Between May 21, 2005, and Dec 1, 2015, patients were followed up for a median of 83 months (IQR 69-94 months). Pegylated interferon alfa-2a induced haematological (66 [80%] of 83 patients) and molecular responses (35 [63%] of 55 patients) in 40 patients with essential thrombocythaemia and 43 patients with polycythaemia vera, with median durations of 66 months (IQR 35-83) and 53 months (24-70), respectively. 26 (39%) of 66 haematological responders and 25 (71%) of 35 molecular responders (with the JAK2 Val617Phe mutation) have maintained some response during follow-up: 49% maintained their best molecular response (nine of ten patients who had a complete response, five of 20 who had a partial response, and three of five who had a minor response). The incidence of major venous-thrombotic events during the study was 1·22 per 100 person-years. Overall, 18 (22%) of 83 patients discontinued therapy due to treatment-related toxicity. Although toxicity rates decreased over time, five patients had treatment-limiting grade 3 or 4 toxicities after 60 months on therapy. 32 patients are still enrolled on the study.
Interpretation: Pegylated interferon alfa-2a can induce durable haematological and molecular responses in patients with essential thrombocythaemia and polycythaemia vera. This drug alone and in combination with other drugs could be explored further in clinical trials.
Funding: US National Cancer Institute.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

Similar articles
-
Symptom burden and quality of life in patients with high-risk essential thrombocythaemia and polycythaemia vera receiving hydroxyurea or pegylated interferon alfa-2a: a post-hoc analysis of the MPN-RC 111 and 112 trials.Lancet Haematol. 2022 Jan;9(1):e38-e48. doi: 10.1016/S2352-3026(21)00343-4. Lancet Haematol. 2022. PMID: 34971581 Free PMC article. Clinical Trial.
-
Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study.Lancet Haematol. 2020 Mar;7(3):e196-e208. doi: 10.1016/S2352-3026(19)30236-4. Epub 2020 Jan 31. Lancet Haematol. 2020. PMID: 32014125 Clinical Trial.
-
Ropeginterferon alfa-2b versus phlebotomy in low-risk patients with polycythaemia vera (Low-PV study): a multicentre, randomised phase 2 trial.Lancet Haematol. 2021 Mar;8(3):e175-e184. doi: 10.1016/S2352-3026(20)30373-2. Epub 2021 Jan 18. Lancet Haematol. 2021. PMID: 33476571 Clinical Trial.
-
Pathophysiology and treatment of platelet-mediated microvascular disturbances, major thrombosis and bleeding complications in essential thrombocythaemia and polycythaemia vera.Platelets. 2004 Mar;15(2):67-84. doi: 10.1080/09537100310001646969. Platelets. 2004. PMID: 15154599 Review.
-
Ropeginterferon alfa-2b for the treatment of patients with polycythemia vera.Drugs Today (Barc). 2020 Mar;56(3):195-202. doi: 10.1358/dot.2020.56.3.3107706. Drugs Today (Barc). 2020. PMID: 32282866 Review.
Cited by
-
Evaluation of Therapeutic Strategies to Reduce the Number of Thrombotic Events in Patients With Polycythemia Vera and Essential Thrombocythemia.Front Oncol. 2021 Feb 16;10:636675. doi: 10.3389/fonc.2020.636675. eCollection 2020. Front Oncol. 2021. PMID: 33665170 Free PMC article. Review.
-
An alternative dosing strategy for ropeginterferon alfa-2b may help improve outcomes in myeloproliferative neoplasms: An overview of previous and ongoing studies with perspectives on the future.Front Oncol. 2023 Jan 19;13:1109866. doi: 10.3389/fonc.2023.1109866. eCollection 2023. Front Oncol. 2023. PMID: 36776307 Free PMC article.
-
Illuminating novel biological aspects and potential new therapeutic approaches for chronic myeloproliferative malignancies.Hematol Oncol. 2020 Dec;38(5):654-664. doi: 10.1002/hon.2771. Epub 2020 Sep 4. Hematol Oncol. 2020. PMID: 32592408 Free PMC article. Review.
-
Complete hematological and major molecular response through treatment with low-dose Interferon alpha 2a in high-risk polycythemia vera patient: a case report.Clin Case Rep. 2021 Oct 6;9(10):e04903. doi: 10.1002/ccr3.4903. eCollection 2021 Oct. Clin Case Rep. 2021. PMID: 34631086 Free PMC article.
-
SOHO State-of-the-Art Update and Next Questions: MPN.Clin Lymphoma Myeloma Leuk. 2018 Jan;18(1):1-12. doi: 10.1016/j.clml.2017.11.008. Clin Lymphoma Myeloma Leuk. 2018. PMID: 29277359 Free PMC article. Review.
References
-
- Dameshek W. Some speculations on the myeloproliferative syndromes [editorial]. Blood. 1951;6(4):372–375. Blood. 2016;127(6):663. - PubMed
-
- Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224–32. - PubMed
-
- Wolanskyj AP, Schwager SM, McClure RF, Larson DR, Tefferi A. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc. 2006;81(2):159–66. - PubMed
-
- Hasselbalch HC, Larsen TS, Riley CH, Jensen MK, Kiladjian JJ. Interferon-alpha in the treatment of Philadelphia-negative chronic myeloproliferative neoplasms. Status and perspectives. Current drug targets. 2011;12(3):392–419. - PubMed
-
- Silver RT. Recombinant interferon-alpha for treatment of polycythaemia vera. Lancet. 1988;2(8607):403. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

