Similarities in the structure of the transcriptional repressor AmtR in two different space groups suggest a model for the interaction with GlnK
- PMID: 28291750
- PMCID: PMC5349308
- DOI: 10.1107/S2053230X17002485
Similarities in the structure of the transcriptional repressor AmtR in two different space groups suggest a model for the interaction with GlnK
Abstract
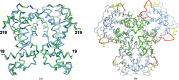
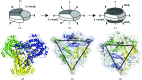
AmtR belongs to the TetR family of transcription regulators and is a global nitrogen regulator that is induced under nitrogen-starvation conditions in Corynebacterium glutamicum. AmtR regulates the expression of transporters and enzymes for the assimilation of ammonium and alternative nitrogen sources, for example urea, amino acids etc. The recognition of operator DNA by homodimeric AmtR is not regulated by small-molecule effectors as in other TetR-family members but by a trimeric adenylylated PII-type signal transduction protein named GlnK. The crystal structure of ligand-free AmtR (AmtRorth) has been solved at a resolution of 2.1 Å in space group P21212. Comparison of its quaternary assembly with the previously solved native AmtR structure (PDB entry 5dy1) in a trigonal crystal system (AmtRtri) not only shows how a solvent-content reduction triggers a space-group switch but also suggests a model for how dimeric AmtR might stoichiometrically interact with trimeric adenylylated GlnK.
Keywords: AmtR adenylylated GlnK complex stoichiometry; Corynebacterium glutamicum; crystal-packing comparison; nitrogen regulation; transcription regulator effector complex.
Figures

Similar articles
-
Crystal structures of adenylylated and unadenylylated PII protein GlnK from Corynebacterium glutamicum.Acta Crystallogr D Struct Biol. 2021 Mar 1;77(Pt 3):325-335. doi: 10.1107/S2059798321000735. Epub 2021 Feb 19. Acta Crystallogr D Struct Biol. 2021. PMID: 33645536 Free PMC article.
-
Structure of AmtR, the global nitrogen regulator of Corynebacterium glutamicum, in free and DNA-bound forms.FEBS J. 2016 Mar;283(6):1039-59. doi: 10.1111/febs.13643. Epub 2016 Feb 10. FEBS J. 2016. PMID: 26744254
-
Crystallization and preliminary crystallographic analysis of the global nitrogen regulator AmtR from Corynebacterium glutamicum.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009 Nov 1;65(Pt 11):1123-7. doi: 10.1107/S174430910903663X. Epub 2009 Oct 30. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009. PMID: 19923732 Free PMC article.
-
Nitrogen control in Corynebacterium glutamicum: proteins, mechanisms, signals.J Microbiol Biotechnol. 2007 Feb;17(2):187-94. J Microbiol Biotechnol. 2007. PMID: 18051748 Review.
-
Application of global analysis techniques to Corynebacterium glutamicum: new insights into nitrogen regulation.J Biotechnol. 2006 Oct 20;126(1):101-10. doi: 10.1016/j.jbiotec.2006.03.039. Epub 2006 May 15. J Biotechnol. 2006. PMID: 16698104 Review.
Cited by
-
Crystal structures of adenylylated and unadenylylated PII protein GlnK from Corynebacterium glutamicum.Acta Crystallogr D Struct Biol. 2021 Mar 1;77(Pt 3):325-335. doi: 10.1107/S2059798321000735. Epub 2021 Feb 19. Acta Crystallogr D Struct Biol. 2021. PMID: 33645536 Free PMC article.
-
Adaptive laboratory evolution accelerated glutarate production by Corynebacterium glutamicum.Microb Cell Fact. 2021 May 10;20(1):97. doi: 10.1186/s12934-021-01586-3. Microb Cell Fact. 2021. PMID: 33971881 Free PMC article.
References
-
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
-
- Amon, J., Titgemeyer, F. & Burkovski, A. (2010). FEMS Microbiol. Rev. 34, 588–605. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

