Adipocyte arrestin domain-containing 3 protein (Arrdc3) regulates uncoupling protein 1 (Ucp1) expression in white adipose independently of canonical changes in β-adrenergic receptor signaling
- PMID: 28291835
- PMCID: PMC5349670
- DOI: 10.1371/journal.pone.0173823
Adipocyte arrestin domain-containing 3 protein (Arrdc3) regulates uncoupling protein 1 (Ucp1) expression in white adipose independently of canonical changes in β-adrenergic receptor signaling
Erratum in
-
Correction: Adipocyte arrestin domain-containing 3 protein (Arrdc3) regulates uncoupling protein 1 (Ucp1) expression in white adipose independently of canonical changes in β-adrenergic receptor signaling.PLoS One. 2017 Jul 10;12(7):e0181492. doi: 10.1371/journal.pone.0181492. eCollection 2017. PLoS One. 2017. PMID: 28700749 Free PMC article.
Abstract
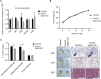
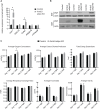
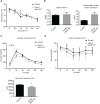
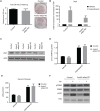
Adaptive thermogenesis and cold-induced activation of uncoupling protein 1 (Ucp1) in brown adipose tissue in rodents is well-described and attributed to sympathetic activation of β-adrenergic signaling. The arrestin domain containing protein Arrdc3 is a regulator of obesity in mice and also appears linked to obesity in humans. We generated a mouse with conditional deletion of Arrdc3, and here we present evidence that genetic ablation of Arrdc3 specifically in adipocytes results in increased Ucp1 expression in subcutaneous and parametrial adipose tissue. Although this increase in expression did not correspond with significant changes in body weight or energy expenditure, adipocyte-specific Arrdc3-null mice had improved glucose tolerance. It was previously hypothesized that Arrdc3 ablation leads to increased β-adrenergic receptor sensitivity; however, in vitro experiments show that Arrdc3-null adipocytes responded to β-adrenergic receptor agonist with decreased Ucp1 levels. Additionally, canonical β-adrenergic receptor signaling was not different in Arrdc3-null adipocytes. These data reveal a role for Arrdc3 in the regulation of Ucp1 expression in adipocytes. However, this adipocyte effect is insufficient to generate the obesity-resistant phenotype of mice with ubiquitous deletion of Arrdc3, indicating a likely role for Arrdc3 in cells other than adipocytes.
Conflict of interest statement
Figures
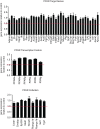
References
-
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, et al. (1997) Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387: 90–94. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

