Long-term Low-Density Lipoprotein Cholesterol-Lowering Efficacy, Persistence, and Safety of Evolocumab in Treatment of Hypercholesterolemia: Results Up to 4 Years From the Open-Label OSLER-1 Extension Study
- PMID: 28291870
- PMCID: PMC5815032
- DOI: 10.1001/jamacardio.2017.0747
Long-term Low-Density Lipoprotein Cholesterol-Lowering Efficacy, Persistence, and Safety of Evolocumab in Treatment of Hypercholesterolemia: Results Up to 4 Years From the Open-Label OSLER-1 Extension Study
Abstract
Importance: The Open-Label Study of Long-term Evaluation Against LDL-C (OSLER-1) evaluated the durability of long-term efficacy and safety during long-term therapy with evolocumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9 (PCSK9).
Objective: To determine whether LDL-C level reductions with evolocumab persist across different populations. Secondary objectives included assessment of adverse events, antidrug antibodies, and factors contributing to treatment discontinuation.
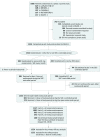
Design, setting, and participants: This ongoing, randomized open-label extension trial (OSLER-1) was conducted at 192 sites in 18 countries. A total of 1324 of 1666 patients randomized into 1 of 5 12-week double-blind phase 2 parent studies completed a parent study and chose to participate in OSLER-1; 1255 received 1 or more evolocumab doses. As of August 2016, 812 of 1324 (61%) had 208 weeks of follow-up. This current study was conducted from October 2011 to August 2016, with a data cutoff of August 26, 2016.
Interventions: During year 1, patients were randomized to evolocumab, 420 mg, plus standard of care (SOC) or SOC alone. After year 1, all patients continuing the study received evolocumab, 420 mg, plus SOC.
Main outcomes and measures: Lipids, safety, and tolerability every 12 weeks. A multivariate model identified factors associated with discontinuation of evolocumab.
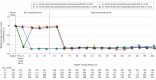
Results: At parent study baseline, the mean (SD) age of the population was 57.1 (11.6) years, with 52.9% being women. The median LDL-C level was 133 mg/dL (to convert to millimoles per liter, multiply by 0.0259). After 52 weeks, evolocumab plus SOC was associated with a significant reduction in LDL-C level by 61% (95% CI, -63% to -60%) vs 2% (95% CI, -5% to -0.2%) with SOC alone (P < .001). At approximately 2, 3, and 4 years of study follow-up, the median LDL-C level was reduced by 59% (95% CI, -60% to -57%), 59% (95% CI, -61% to -58%), and 57% (95% CI, -59% to -55%), respectively, from parent study baseline. For patients receiving statin therapy unchanged from baseline, at week 208, the median LDL-C level reduction was 58%. No neutralizing antibodies to evolocumab were detected. The annualized incidence of new-onset diabetes was 4% in the SOC alone group and, adjusting for duration of evolocumab exposure, 2.8% in the evolocumab plus SOC group. Neurocognitive event rates were 0% (SOC alone) and 0.4% (evolocumab plus SOC). A total of 79% of patients persisted with evolocumab treatment, with a mean exposure duration of 44 months.
Conclusions and relevance: In the longest clinical trial exposure to a PCSK9 inhibitor to date, evolocumab produced sustained reductions in LDL-C levels. The annual frequency of adverse events did not occur more frequently with cumulative exposure during open-label observation.
Trial registration: clinicaltrials.gov Identifier: NCT01439880.
Conflict of interest statement
Figures
Comment in
-
Factors Affecting the Cost of Airplanes and Use of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors.JAMA Cardiol. 2017 Jun 1;2(6):596-597. doi: 10.1001/jamacardio.2017.0760. JAMA Cardiol. 2017. PMID: 28291865 No abstract available.
-
PCSK9 Inhibitors, Statins, Low-Density Lipoprotein Cholesterol, Mevalonate Pathway, and Toxicity-Reply.JAMA Cardiol. 2017 Oct 1;2(10):1169. doi: 10.1001/jamacardio.2017.2734. JAMA Cardiol. 2017. PMID: 28813546 No abstract available.
-
PCSK9 Inhibitors, Statins, Low-Density Lipoprotein Cholesterol, Mevalonate Pathway, and Toxicity.JAMA Cardiol. 2017 Oct 1;2(10):1168-1169. doi: 10.1001/jamacardio.2017.2727. JAMA Cardiol. 2017. PMID: 28813549 No abstract available.
-
Cholesterol is the Cause of Atherosclerosis.Am J Cardiol. 2017 Nov 1;120(9):1696. doi: 10.1016/j.amjcard.2017.07.071. Epub 2017 Aug 1. Am J Cardiol. 2017. PMID: 28847597 No abstract available.
References
-
- Stroes ES, Thompson PD, Corsini A, et al. ; European Atherosclerosis Society Consensus Panel . Statin-associated muscle symptoms: impact on statin therapy: European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. - PMC - PubMed
-
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients: the PRIMO Study. Cardiovasc Drugs Ther. 2005;19(6):403-414. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

