Highly efficient mesophyll protoplast isolation and PEG-mediated transient gene expression for rapid and large-scale gene characterization in cassava (Manihot esculenta Crantz)
- PMID: 28292294
- PMCID: PMC5351281
- DOI: 10.1186/s12896-017-0349-2
Highly efficient mesophyll protoplast isolation and PEG-mediated transient gene expression for rapid and large-scale gene characterization in cassava (Manihot esculenta Crantz)
Abstract
Background: Cassava (Manihot esculenta Crantz) is a major crop extensively cultivated in the tropics as both an important source of calories and a promising source for biofuel production. Although stable gene expression have been used for transgenic breeding and gene function study, a quick, easy and large-scale transformation platform has been in urgent need for gene functional characterization, especially after the cassava full genome was sequenced.
Methods: Fully expanded leaves from in vitro plantlets of Manihot esculenta were used to optimize the concentrations of cellulase R-10 and macerozyme R-10 for obtaining protoplasts with the highest yield and viability. Then, the optimum conditions (PEG4000 concentration and transfection time) were determined for cassava protoplast transient gene expression. In addition, the reliability of the established protocol was confirmed for subcellular protein localization.
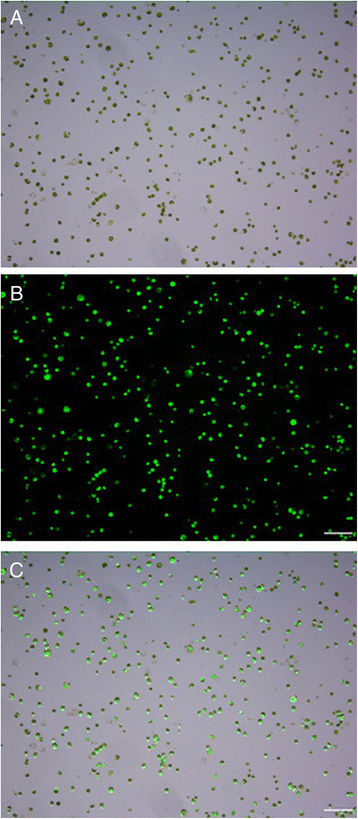
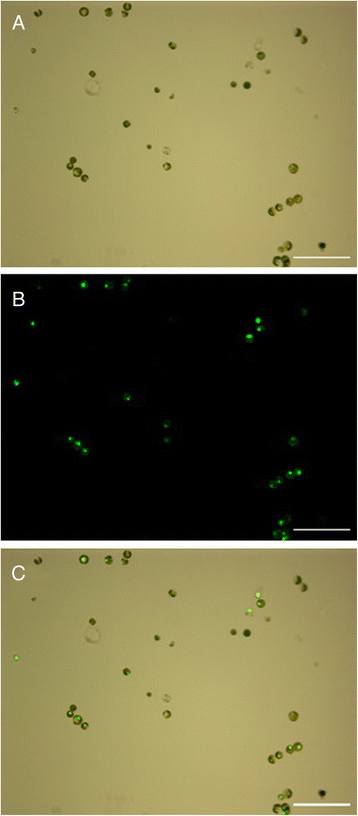
Results: In this work we optimized the main influencing factors and developed an efficient mesophyll protoplast isolation and PEG-mediated transient gene expression in cassava. The suitable enzyme digestion system was established with the combination of 1.6% cellulase R-10 and 0.8% macerozyme R-10 for 16 h of digestion in the dark at 25 °C, resulting in the high yield (4.4 × 107 protoplasts/g FW) and vitality (92.6%) of mesophyll protoplasts. The maximum transfection efficiency (70.8%) was obtained with the incubation of the protoplasts/vector DNA mixture with 25% PEG4000 for 10 min. We validated the applicability of the system for studying the subcellular localization of MeSTP7 (an H+/monosaccharide cotransporter) with our transient expression protocol and a heterologous Arabidopsis transient gene expression system.
Conclusion: We optimized the main influencing factors and developed an efficient mesophyll protoplast isolation and transient gene expression in cassava, which will facilitate large-scale characterization of genes and pathways in cassava.
Keywords: Cassava; Manihot esculenta; Protoplast isolation; Subcellular localization; Transient expression system.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

