Robust Generation of Quiescent Porcine Valvular Interstitial Cell Cultures
- PMID: 28292746
- PMCID: PMC5524027
- DOI: 10.1161/JAHA.116.005041
Robust Generation of Quiescent Porcine Valvular Interstitial Cell Cultures
Abstract
Background: Valvular interstitial cells (VICs) in the healthy aortic valve leaflet exhibit a quiescent phenotype, with <5% of VICs exhibiting an activated phenotype. Yet, in vitro culture of VICs on tissue culture polystyrene surfaces in standard growth medium results in rapid transformation to an activated phenotype in >90% of cells. The inability to preserve a healthy VIC phenotype during in vitro studies has hampered the elucidation of mechanisms involved in calcific aortic valve disease. This study describes the generation of quiescent populations of porcine VICs in 2-dimensional in vitro culture and their utility in studying valve pathobiology.
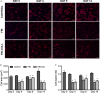
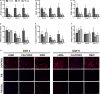
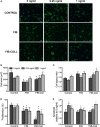
Methods and results: Within 4 days of isolation from fresh porcine hearts, VICs cultured in standard growth conditions were predominantly myofibroblastic (activated VICs). This myofibroblastic phenotype was partially reversed within 4 days, and fully reversed within 9 days, following application of a combination of a fibroblast media formulation with culture on collagen coatings. Specifically, culture in this combination significantly reduced several markers of VIC activation, including proliferation, apoptosis, α-smooth muscle actin expression, and matrix production, relative to standard growth conditions. Moreover, VICs raised in a fibroblast media formulation with culture on collagen coatings exhibited dramatically increased sensitivity to treatment with transforming growth factor β1, a known pathological stimulus, compared with VICs raised in either standard culture or medium with a fibroblast media formulation.
Conclusions: The approach using a fibroblast media formulation with culture on collagen coatings generates quiescent VICs that more accurately mimic a healthy VIC population and thus has the potential to transform the study of the mechanisms of VIC activation and dysfunction involved in the early stages of calcific aortic valve disease.
Keywords: aortic valve; calcific aortic valve disease; differentiation; myofibroblasts; quiescence; valvular interstitial cell.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures


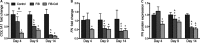
Similar articles
-
Transforming growth factor-β1 promotes fibrosis but attenuates calcification of valvular tissue applied as a three-dimensional calcific aortic valve disease model.Am J Physiol Heart Circ Physiol. 2020 Nov 1;319(5):H1123-H1141. doi: 10.1152/ajpheart.00651.2019. Epub 2020 Sep 28. Am J Physiol Heart Circ Physiol. 2020. PMID: 32986963
-
Simulation of early calcific aortic valve disease in a 3D platform: A role for myofibroblast differentiation.J Mol Cell Cardiol. 2016 May;94:13-20. doi: 10.1016/j.yjmcc.2016.03.004. Epub 2016 Mar 17. J Mol Cell Cardiol. 2016. PMID: 26996755 Free PMC article.
-
Secreted Factors From Proinflammatory Macrophages Promote an Osteoblast-Like Phenotype in Valvular Interstitial Cells.Arterioscler Thromb Vasc Biol. 2020 Nov;40(11):e296-e308. doi: 10.1161/ATVBAHA.120.315261. Epub 2020 Sep 17. Arterioscler Thromb Vasc Biol. 2020. PMID: 32938214 Free PMC article.
-
Mechanoregulation of aortic valvular interstitial cell life and death.J Long Term Eff Med Implants. 2015;25(1-2):3-16. doi: 10.1615/jlongtermeffmedimplants.2015011759. J Long Term Eff Med Implants. 2015. PMID: 25955003 Review.
-
Valve Interstitial Cells: The Key to Understanding the Pathophysiology of Heart Valve Calcification.J Am Heart Assoc. 2017 Sep 14;6(9):e006339. doi: 10.1161/JAHA.117.006339. J Am Heart Assoc. 2017. PMID: 28912209 Free PMC article. Review. No abstract available.
Cited by
-
Angiogenic Secretion Profile of Valvular Interstitial Cells Varies With Cellular Sex and Phenotype.Front Cardiovasc Med. 2021 Aug 30;8:736303. doi: 10.3389/fcvm.2021.736303. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34527715 Free PMC article.
-
Identifying molecular and functional similarities and differences between human primary cardiac valve interstitial cells and ventricular fibroblasts.Front Bioeng Biotechnol. 2023 Mar 27;11:1102487. doi: 10.3389/fbioe.2023.1102487. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37051268 Free PMC article.
-
Interstitial cells in calcified aortic valves have reduced differentiation potential and stem cell-like properties.Sci Rep. 2019 Sep 10;9(1):12934. doi: 10.1038/s41598-019-49016-0. Sci Rep. 2019. PMID: 31506459 Free PMC article.
-
On Valve Interstitial Cell Signaling: The Link Between Multiscale Mechanics and Mechanobiology.Cardiovasc Eng Technol. 2021 Feb;12(1):15-27. doi: 10.1007/s13239-020-00509-4. Epub 2021 Feb 1. Cardiovasc Eng Technol. 2021. PMID: 33527256 Free PMC article. Review.
-
A three-dimensional valve-on-chip microphysiological system implicates cell cycle progression, cholesterol metabolism and protein homeostasis in early calcific aortic valve disease progression.Acta Biomater. 2024 Sep 15;186:167-184. doi: 10.1016/j.actbio.2024.07.036. Epub 2024 Jul 30. Acta Biomater. 2024. PMID: 39084496
References
-
- Chen JH, Simmons CA. Cell‐matrix interactions in the pathobiology of calcific aortic valve disease: critical roles for matricellular, matricrine, and matrix mechanics cues. Circ Res. 2011;108:1510–1524. - PubMed
-
- Rajamannan NM, Evans FJ, Aikawa E, Grande‐Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O'Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: calcific aortic valve disease‐2011 update. Circulation. 2011;124:1783–1791. - PMC - PubMed
-
- Li C, Xu S, Gotlieb AI. The progression of calcific aortic valve disease through injury, cell dysfunction, and disruptive biologic and physical force feedback loops. Cardiovasc Pathol. 2013;22:1–8. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

