Nonlinear Modulation of Cutaneous Reflexes with Increasing Speed of Locomotion in Spinal Cats
- PMID: 28292829
- PMCID: PMC6596719
- DOI: 10.1523/JNEUROSCI.3042-16.2017
Nonlinear Modulation of Cutaneous Reflexes with Increasing Speed of Locomotion in Spinal Cats
Abstract
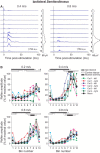
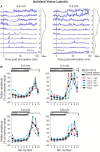
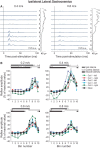
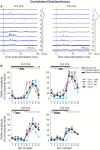
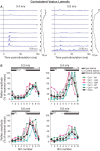
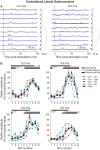
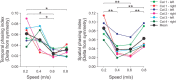
Cutaneous reflexes are important for responding rapidly to perturbations, correcting limb trajectory, and strengthening support. During locomotion, they are modulated by phase to generate functionally appropriate responses. The goal of the present study was to determine whether cutaneous reflexes and their phase-dependent modulation are altered with increasing speed and if this is accomplished at the spinal level. Four adult cats that recovered stable hindlimb locomotion after spinal transection were implanted with electrodes to record hindlimb muscle activity chronically and to stimulate the superficial peroneal nerve electrically to evoke cutaneous reflexes. The speed-dependent modulation of cutaneous reflexes was assessed by evoking and characterizing ipsilateral and contralateral responses in semitendinosus, vastus lateralis, and lateral gastrocnemius muscles at four treadmill speeds: 0.2, 0.4, 0.6, and 0.8 m/s. The amplitudes of ipsilateral and contralateral responses were largest at intermediate speeds of 0.4 and 0.6 m/s, followed by the slowest and fastest speeds of 0.2 and 0.8 m/s, respectively. The phase-dependent modulation of reflexes was maintained across speeds, with ipsilateral and contralateral responses peaking during the stance-to-swing transition and swing phase of the ipsilateral limb or midstance of the contralateral limb. Reflex modulation across speeds also correlated with the spatial symmetry of the locomotor pattern, but not with temporal symmetry. That the cutaneous reflex amplitude in all muscles was similarly modulated with increasing speed independently of the background level of muscle activity is consistent with a generalized premotoneuronal spinal control mechanism that could help to stabilize the locomotor pattern when changing speed.SIGNIFICANCE STATEMENT When walking, receptors located in the skin respond to mechanical pressure and send signals to the CNS to correct the trajectory of the limb and to reinforce weight support. These signals produce different responses, or reflexes, if they occur when the foot is contacting the ground or in the air. This is known as phase-dependent modulation of reflexes. However, when walking at faster speeds, we do not know if and how these reflexes are changed. In the present study, we show that reflexes from the skin are modulated with speed and that this is controlled at the level of the spinal cord. This modulation could be important in preventing sensory signals from destabilizing the walking pattern.
Keywords: cutaneous reflex; locomotion; speed; spinal cord.
Copyright © 2017 the authors 0270-6474/17/373896-17$15.00/0.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
