Embryoids, organoids and gastruloids: new approaches to understanding embryogenesis
- PMID: 28292844
- PMCID: PMC5358114
- DOI: 10.1242/dev.143529
Embryoids, organoids and gastruloids: new approaches to understanding embryogenesis
Abstract
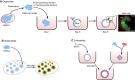
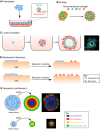
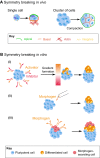
Cells have an intrinsic ability to self-assemble and self-organize into complex and functional tissues and organs. By taking advantage of this ability, embryoids, organoids and gastruloids have recently been generated in vitro, providing a unique opportunity to explore complex embryological events in a detailed and highly quantitative manner. Here, we examine how such approaches are being used to answer fundamental questions in embryology, such as how cells self-organize and assemble, how the embryo breaks symmetry, and what controls timing and size in development. We also highlight how further improvements to these exciting technologies, based on the development of quantitative platforms to precisely follow and measure subcellular and molecular events, are paving the way for a more complete understanding of the complex events that help build the human embryo.
Keywords: Early development; Patterning; Self-assembly; Self-organization; Symmetry breaking; Tissue mechanics.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures
References
-
- Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K. and Walter P. (2014). Molecular Biology of the Cell. New York: Garland Science.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

