Lung organoids: current uses and future promise
- PMID: 28292845
- PMCID: PMC5358104
- DOI: 10.1242/dev.140103
Lung organoids: current uses and future promise
Abstract
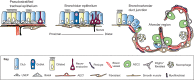
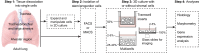
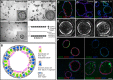
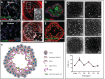
Lungs are composed of a system of highly branched tubes that bring air into the alveoli, where gas exchange takes place. The proximal and distal regions of the lung contain epithelial cells specialized for different functions: basal, secretory and ciliated cells in the conducting airways and type II and type I cells lining the alveoli. Basal, secretory and type II cells can be grown in three-dimensional culture, with or without supporting stromal cells, and under these conditions they give rise to self-organizing structures known as organoids. This Review summarizes the different methods for generating organoids from cells isolated from human and mouse lungs, and compares their final structure and cellular composition with that of the airways or alveoli of the adult lung. We also discuss the potential and limitations of organoids for addressing outstanding questions in lung biology and for developing new drugs for disorders such as cystic fibrosis and asthma.
Keywords: Lung organoids; Lung progenitors; Plasticity; Stem cells.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures
References
-
- Butler C. R., Hynds R. E., Gowers K. H. C., Lee D. D. H., Brown J. M., Crowley C., Teixeira V. H., Smith C. M., Urbani L., Hamilton N. J. et al. (2016). Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am. J. Respir. Crit. Care Med. 194, 156-168. 10.1164/rccm.201507-1414OC - DOI - PMC - PubMed
-
- Chen H., Matsumoto K., Brockway B. L., Rackley C. R., Liang J., Lee J.-H., Jiang D., Noble P. W., Randell S. H., Kim C. F. et al. (2012). Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells 30, 1948-1960. 10.1002/stem.1150 - DOI - PMC - PubMed
-
- Chu H. W., Rios C., Huang C., Wesolowska-Andersen A., Burchard E. G., O'Connor B. P., Fingerlin T. E., Nichols D., Reynolds S. D. and Seibold M. A. (2015). CRISPR-Cas9-mediated gene knockout in primary human airway epithelial cells reveals a proinflammatory role for MUC18. Gene Ther. 22, 822-829. 10.1038/gt.2015.53 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

